
Focus on Innovation
George Nakayama
President and CEO
Daiichi Sankyo Co., LTD.
JP Morgan Healthcare Conference 2016

1
Forward-Looking Statements
Financial forecasts, future projections and R&D information
that Daiichi Sankyo discloses may include information that
might be classified as “Forward-Looking Statement”.
These forward-looking statements represent our current
assumptions based on information currently available.
Please note that such are subject to a number of known
and unknown risks and uncertainties and our future
performance may differ from the expectations as expressed
in such statements.

Major Management Topics
Background
Edoxaban
Japan Business
US Business
R&D Topics (Oncology Strategy)
Conclusion
2
Today’s Agenda

Major Management Topics
Background
Edoxaban
Japan Business
US Business
R&D Topics (Oncology Strategy)
Conclusion
3
Today’s Agenda
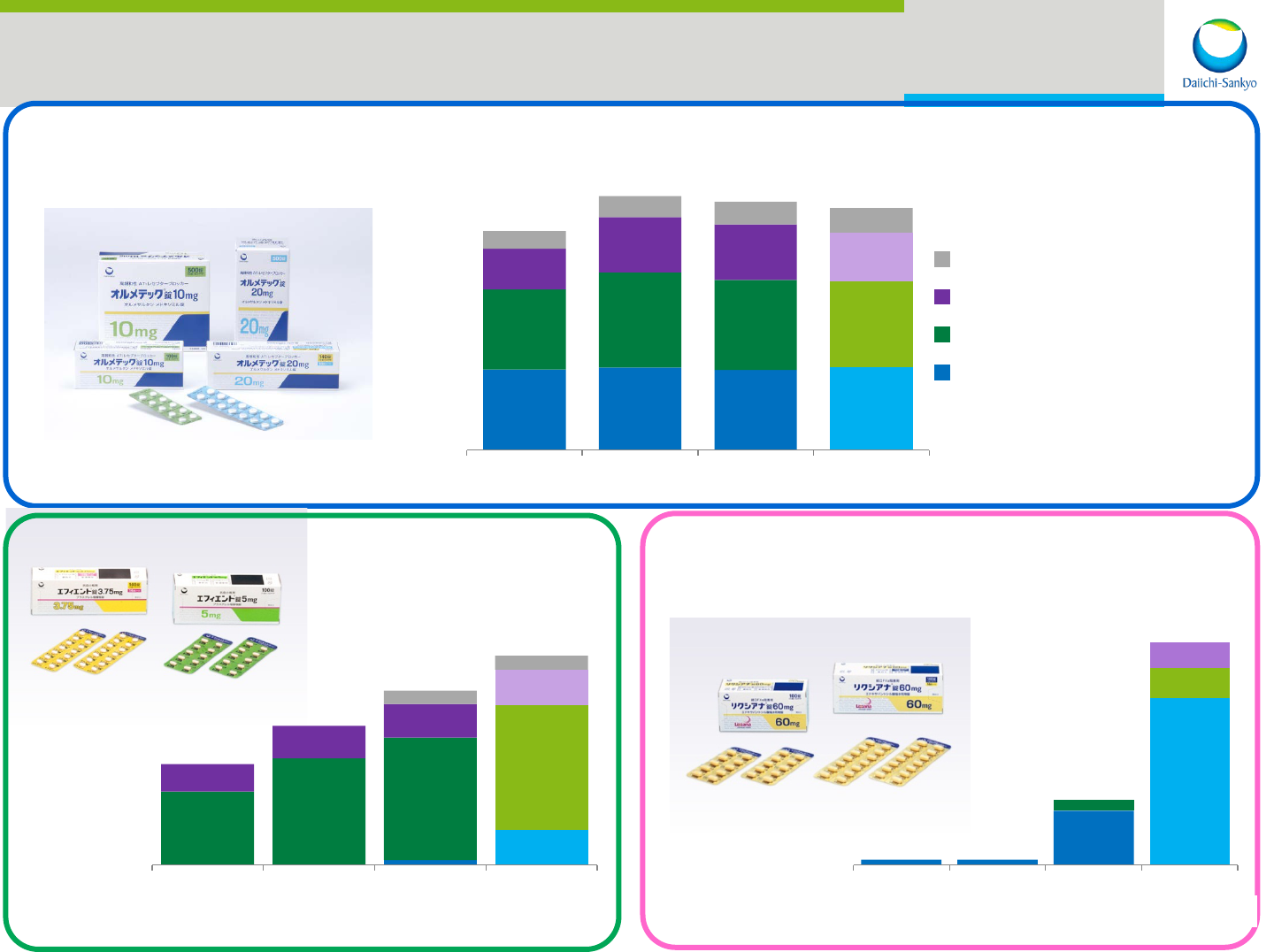
Major Global Products
*Currency rate : JPY/USD=120.0
(USD M)
793
813
789
817
790
936
888
842
401
547
544
483
173
206
225
242
2,157
2,502
2,446
2,384
FY2012 FY2013 FY2014
FY2015
(
Estimate
)
Others
EU
US
Japan
anti-hypertension
Olmesartan
(Benicar: US Olmetec: JP, EU)
6
42
88
128
147
33
39
40
16
121
167
209
FY2012 FY2013 FY2014
FY2015
(
estimate
)
antiplatelet
Prasugrel
(Effient: US Efient: JP, EU)
30
92
6
17
14
3
3
36
123
FY2012 FY2013 FY2014
FY2015
(
Plan
)
anticoagulant
Edoxaban
(Savaysa: US Lixiana: JP, EU)
Not
disclose
Not
disclose
4
(estimate)
(estimate)
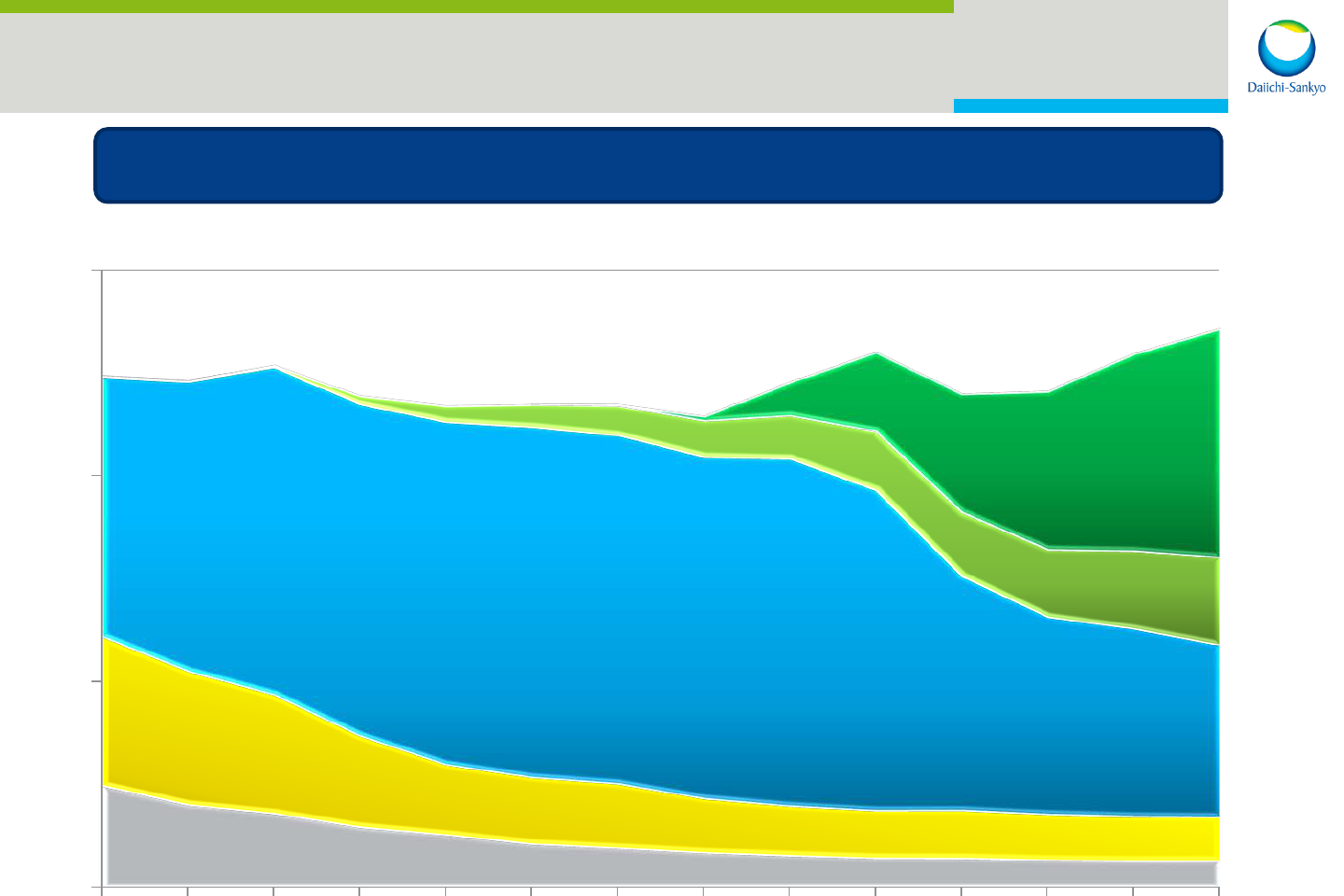
5
Olmesartan
Benicar, Olmetec etc
Prasugrel
Effient, Efient
Edoxaban
Lixiana, Savaysa
Levofloxacin
Pravastatin
FY2007 FY2012 FY2016 FY2020
*The above figures do not include partner sales
Management Focus
Sustainable growth with smooth transition of key drivers
6
Management Focus (cont.)
Global
Japan
US
Launch Edoxaban (Lixiana/Savaysa) & maximize
its potential as a flag-ship product
Achieve No. 1 market share by maximizing new
drugs
Efient (Effient), Memary (Namenda), Nexium,
Pralia (Prolia), Ranmark (Xgeva) etc.
Lacosamide (Vimpat)
Achieve rapid growth of new drugs & establish
core franchises
DSI: Movantik, CL-108, Mirogabalin Pain
Tivantinib, Pexidartinib, Quizartinib Oncology
LPI: Venofer, Injectafer IV Iron franchise
Strategies for growth beyond Olmesartan LOE

Major Management Topics
Background
Edoxaban
Japan Business
US Business
R&D Topics (Oncology Strategy)
Conclusion
7
Today’s Agenda

8
Edoxaban: Competitive Advantage
Oral, highly selective, direct, and reversible Factor Xa inhibitor
The only once-daily NOAC that offers superiority compared to
warfarin with less major bleeding in non-valvular atrial fibrillation
and with less clinically relevant bleeding in DVT/PE
The only NOAC in Japan with three approved major indications :
AF, VTE and DVT-OS
AF: Atrial Fibrillation; VTE: Venous Thromboembolism; DVT-OS: Deep Vein Thrombosis - Orthopedic Surgery
9
Edoxaban: Major Regions
Launched for DVT-OS in 2011
Launched for AF and VTE in 2014
Sales growing steadily
Revenue target: ~USD 100mn in FY2015
Launched for AF and VTE in 2015
AF: indicated only for patients with CrCL ≤95 mL/min
VTE: without limitation of use
Ongoing negotiations with payers
Approved for AF and VTE by EC without limitation of use in 2015
Recommended for VTE and AF by NICE
Good start in each country
US
JPN
EU
10
Switzerland: May 2015
UK: Jul. 2015
Germany: Aug. 2015
Ireland: Sep. 2015
The Netherlands: Nov. 2015
(Launch Dates)
Japan
DVT-OS: 2011
AF, VTE: 2014
(Launch)
US: Feb. 2015
(Launch Date)
Global revenue: USD 36M (FY2014)→USD 123M (FY2015 Plan)
*Currency rate : JPY/USD=120.0
Edoxaban: Global Development
(Approved)
Korea: Aug. 2015
(NDA)
China, Hong Kong,
Taiwan, Thailand,
Australia, Brazil,
Canada

Major Management Topics
Background
Edoxaban
Japan Business
US Business
R&D Topics (Oncology Strategy)
Conclusion
11
Today’s Agenda
12
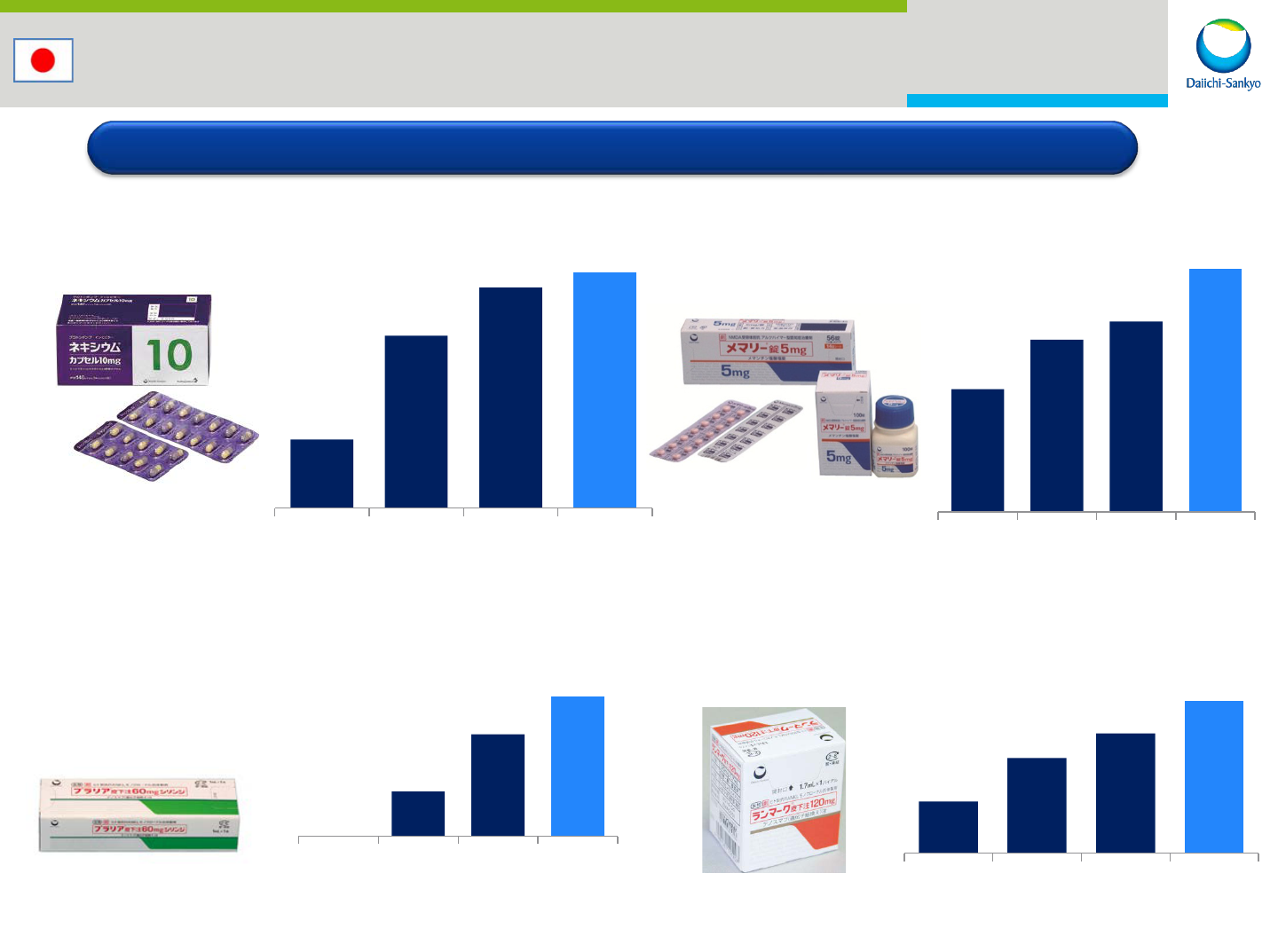
anti-ulcer(PPI)
Nexium
treatment for alzheimer
Memary (Namenda)
treatment for osteoprosis
Pralia (Prolia)
(Plan)
180
452
578
617
2012 2013 2014 2015
198
278
307
392
2012 2013 2014 2015
27
61
83
2012 2013 2014 2015
37
68
85
108
2012 2013 2014 2015
treatment for bone metastasis
Ranmark (Xgeva)
(Plan)
(Plan)
(Plan)
Japan: Major Products
(USD M)
*Currency rate : JPY/USD=120.0
For No.1 market share company in Japan
12
(estimate)
(estimate)
(estimate)
(estimate)
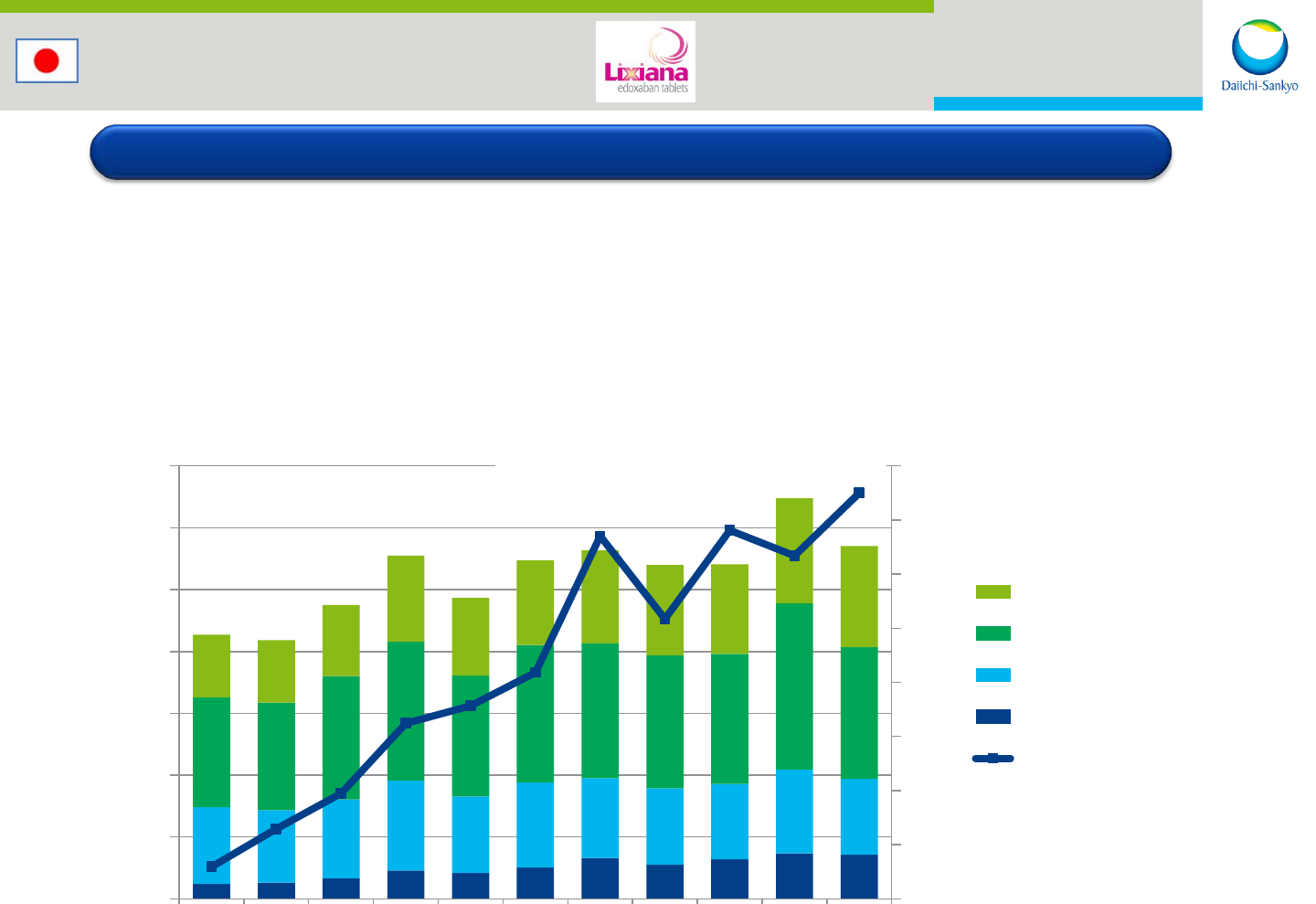
Japan: Edoxaban
13
Launched for DVT-OS in 2011
Launched for AF and VTE in 2014
Revenue target: ~USD 100mn in FY2015
5
6
7
8
9
10
11
12
13
0
2,000
4,000
6,000
8,000
10,000
12,000
14,000
Jan-15 Feb-15 Mar-15 Apr-15 May-15 Jun-15 Jul-15 Aug-15 Sep-15 Oct-15 Nov-15
Product C
Product B
Product A
Lixiana
Lixiana (%)
M JPY (%)
©2015 IMSHealth
Calculated based on JPM 2015 Jan-Nov
Reprinted with permission
Market Share: 12.5%
Steady sales growth

Major Management Topics
Background
Edoxaban
Japan Business
US Business
R&D Topics (Oncology Strategy)
Conclusion
14
Today’s Agenda
United States: Daiichi Sankyo
15
Two Subsidiaries in US with marketing functions
• HQ Location: Parsippany, New Jersey
• Other function: Research, development and packaging
• HQ Location: Shirley, New York
• Other function: Research, development and manufacturing
DSI: Daiichi Sankyo, Inc.
LPI: Luitpold Pharmaceuticals, Inc.

United States: Daiichi Sankyo
16
• HQ Location: Parsippany, New Jersey
• Other function: Research, development and packaging
DSI: Daiichi Sankyo, Inc.
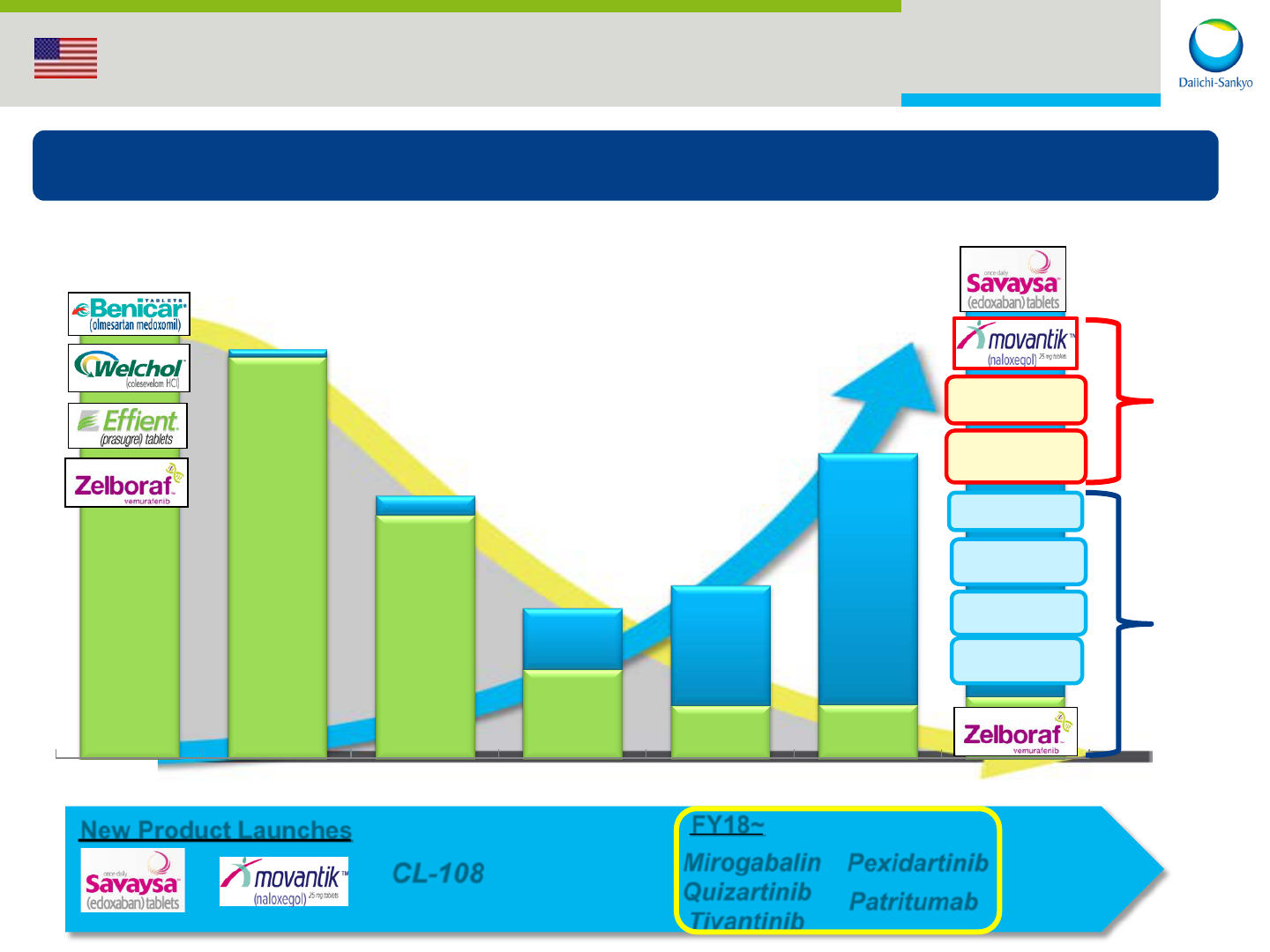
17
FY14 FY15 FY16 FY17 FY18 FY19 FY20
New Product Launches
Mirogabalin
Quizartinib
Tivantinib
Pexidartinib
Patritumab
FY18~
CL-108
CL-108
Mirogabalin
Tivantinib
Quizartinib
Patritumab
Pexidartinib
Pain
Oncology
United States: DSI Portfolio
To specialty products in CV, Pain and Oncology
New products
Current products

18
United States: DSI (cont.)
Pain franchise: Near-term opportunities
Three products targeting important, but unmet needs
CL-108
Mirogabalin
Opioid-induced
Constipation (OIC) for
patients taking opioids for chronic,
non-cancer related pain
Launched in U.S. May 2015
Pain and Opioid-Induced Nausea &
Vomiting (OINV)
Phase 3 completed
Pain Associated with Fibromyalgia
Phase 3 ongoing, TLR* anticipated in 1H 2017
*Co-Commercialized by AstraZeneca & Daiichi Sankyo, Inc.
*Top line results

LPI: A Diversified Specialty Company
19
IRON FRANCHISE
(> 50% share of non HD segment)
GENERIC
INJECTABLE
FRANCHISE
Competitive among high value specialty-branded &
generic injectable market segments
Venofer
®
Injectafer
®
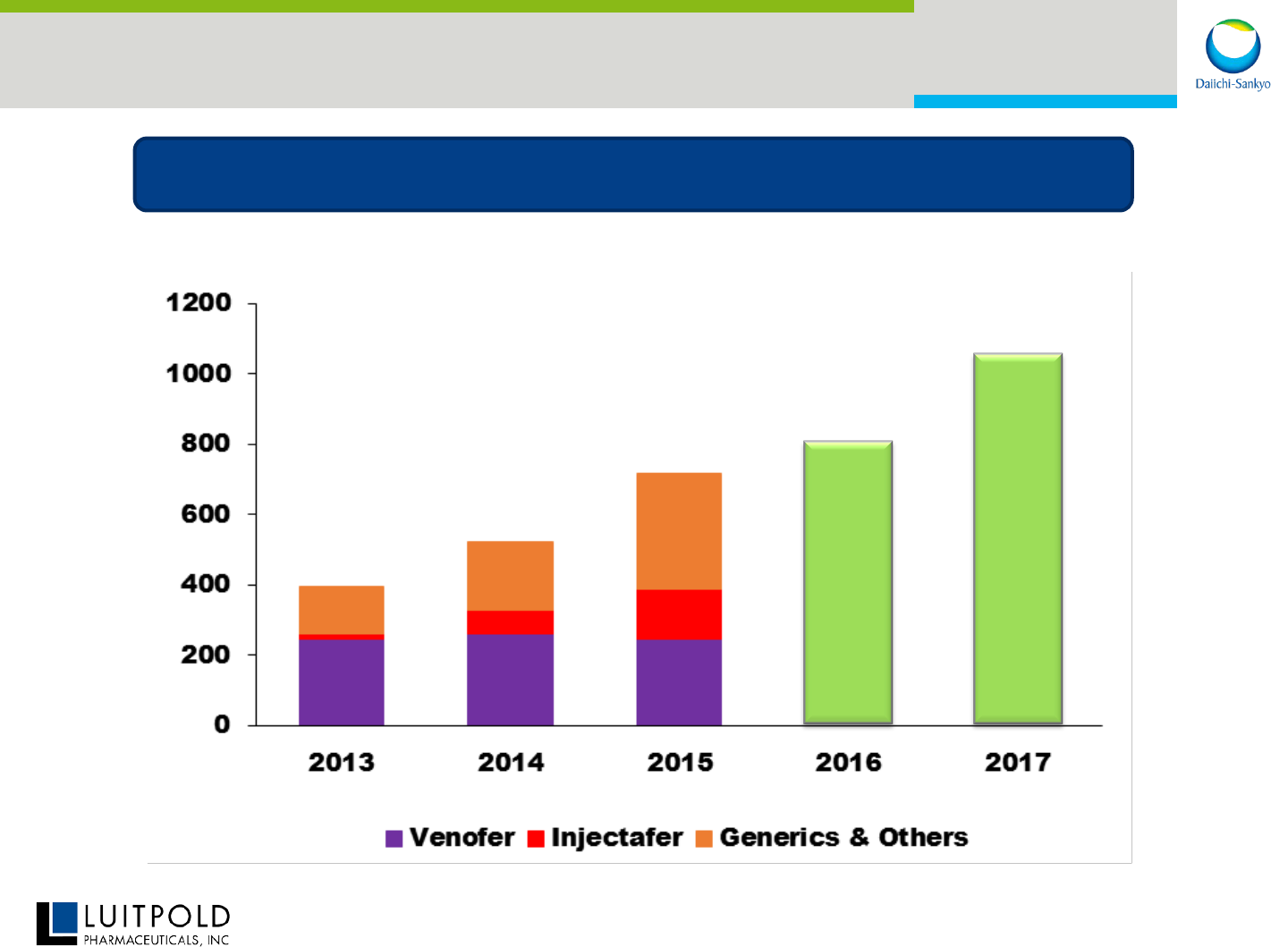
LPI: Positioned for Accelerated Growth
20
Double-digit revenue and profit growth
*Based on forecast
($M)$
LE
395
522
714
*
*
*

Major Management Topics
Background
Edoxaban
Japan Business
US Business
R&D Topics (Oncology Strategy)
Conclusion
21
Today’s Agenda
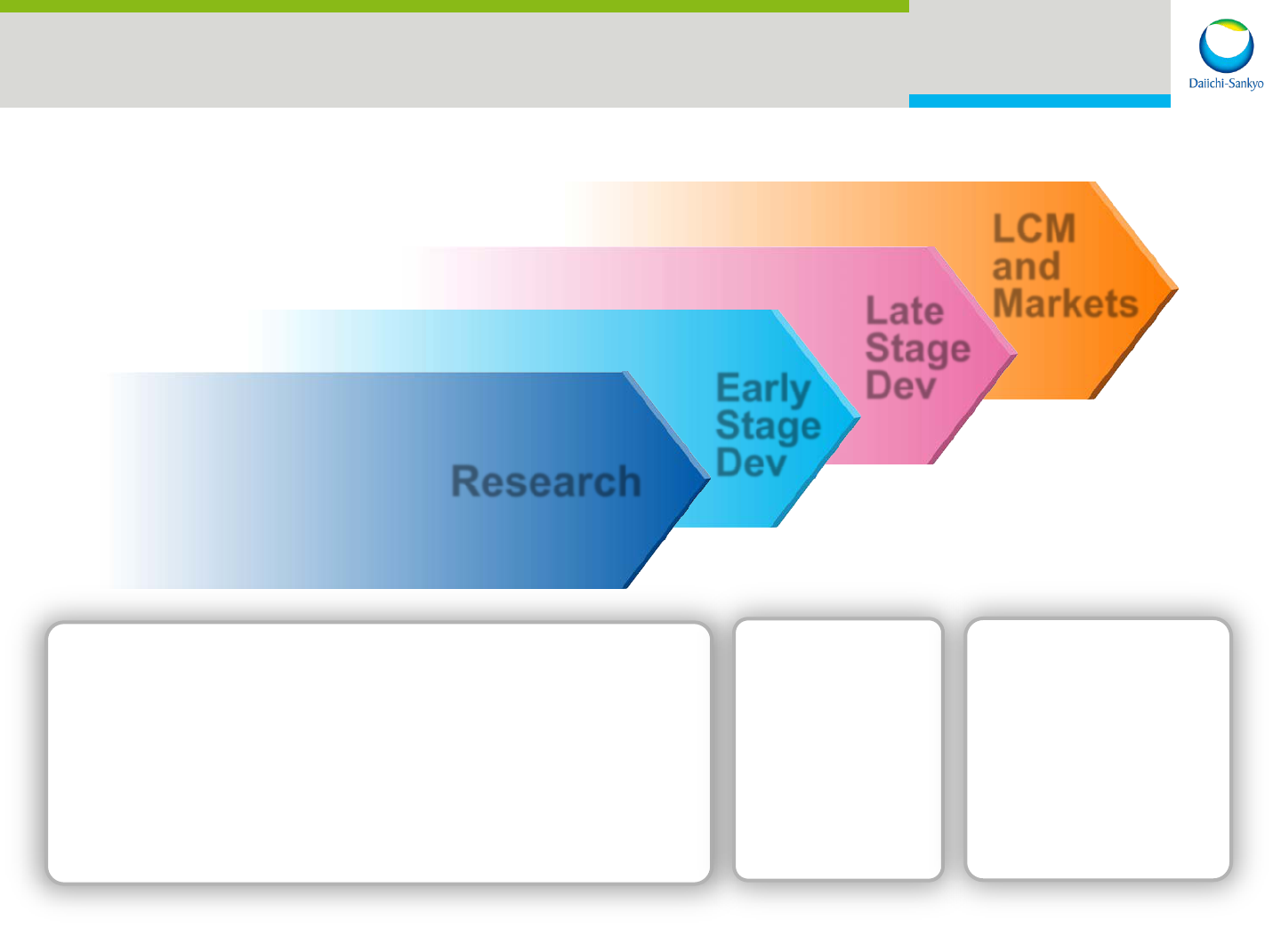
22
R&D: Focus Therapeutic Areas
Priority Areas for Discovery*
- Oncology
- Cardiovascular-Metabolics
- Pain
Research
Early
Stage
Dev
Late
Stage
Dev
LCM
and
Markets
Oncology
CV-M
Pain
Thrombosis
Hypertension
Pain
*Discovery:Research and Early Development up to Proof of Concept
22
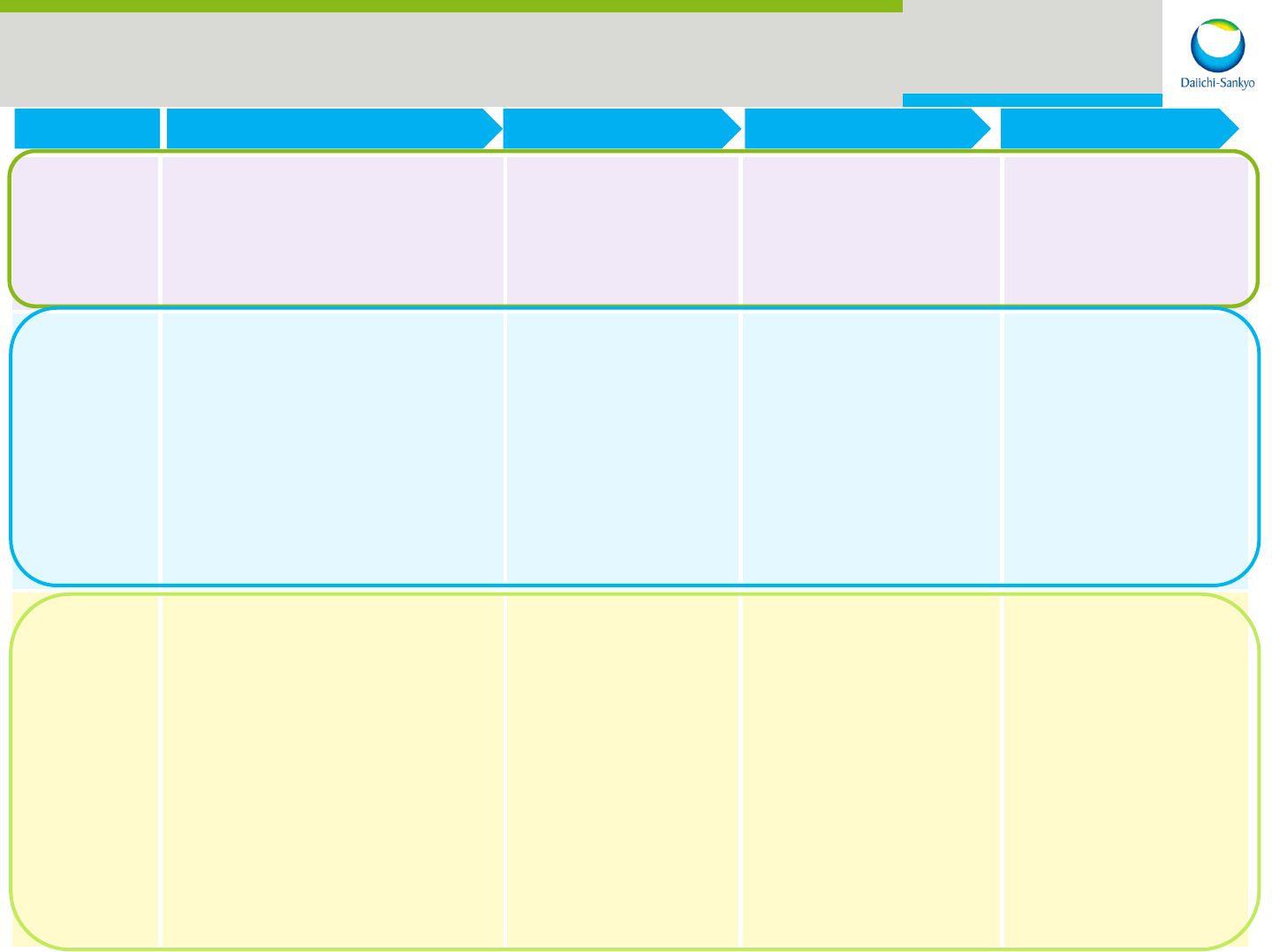
23
Phase 1 Phase 2
Phase 3 Application
Therapeutic
area
Major R&D Pipeline As of October 2015
Cardio
vascular-
Metabolics
DS-1040
(Acute ischemic stroke / TAFIa inhibitor)
DS-8312
(Hypertriglyceridemia)
DS-2330
(Hyperphosphatemia
)
DS-9231/TS23
(Thrombosis / α2-PI inactivating antibody)
CS-3150 (JP)
(Hypertension
・
DM nephropathy /
MR antagonist)
DS-8500 (JP)
(Diabetes / GPR119 agonist)
Prasugrel (JP)
(CS-747 / ischemic stroke / anti-
platelet agent)
Prasugrel (US)
(CS-747 / sickle cell disease / anti-
platelet agent)
Edoxaban (ASCA etc.)
(DU-176b / AF / oral factor Xa
inhibitor)
Edoxaban (ASCA etc.)
(DU-176b / VTE / oral factor Xa
inhibitor)
Oncology
DS-3032 (US/JP)
(MDM2 inhibitor)
PLX7486 (US)
(FMS / TRK inhibitor)
PLX8394 (US)
(BRAF inhibitor)
DS-6051 (US)
(NTRK/ROS1 inhibitor)
PLX9486 (US)
(KIT inhibitor)
Patritumab (US/EU)
(U3-1287 / anti-HER3 antibody)
Pexidartinib (US)
(PLX3397
/ FMS/KIT/FLT3-ITD
inhibitor)
Tivantinib (US/EU)
(ARQ 197 / HCC / MET inhibitor)
Denosumab (JP)
(AMG 162 / breast cancer adjuvant /
anti-RANKL antibody)
Nimotuzumab (JP)
(DE-766 / gastric cancer / anti-EGFR
antibody)
Vemurafenib (US/EU)
(PLX4032 / melanoma adjuvant / BRAF
inhibitor)
Quizartinib (US/EU)
(AC220 / AML / FLT3-ITD inhibitor)
Pexidartinib (US/EU)
(PLX3397/TGCT / FMS/KIT/FLT3-ITD
inhibitor)
Others
DS-1093
(Anemia of chronic kidney disease
/ HIF-PH inhibitor)
DS-3801
(Chronic obstipation / GPR38 agonist)
DS-1971
(Chronic pain)
DS-1501
(Osteoporosis / Anti-Siglec-15 antibody)
DS-7080
(AMD / Angiogenesis inhibitor)
VN-0102/JVC-001 (JP)
(MMR vaccine)
SUN13837 (US/EU)
(Spinal cord injury / modulator of
bFGF signaling system)
Laninamivir (US/EU)
(CS-8958 / anti-influenza /
out-licensing with Biota)
Mirogabalin (US/EU)
(DS-5565 / fibromyalgia / α2δ ligand)
Mirogabalin (JP/Asia)
(DS-5565 / DPNP/ α2δ ligand)
Mirogabalin (JP/Asia)
(DS-5565 / PHN / α2δ ligand)
Denosumab (JP)
(AMG 162 / rheumatoid arthritis /
anti-RANKL anti-body)
Hydromorphone (JP)
(DS-7113 / cancer pain /
opioid μ-receptor regulator)
CHS-0214 (JP)
(Etanercept BS / rheumatoid
arthritis / TNFα inhibitor)
CL-108 (US)
(Acute pain / opioid μ-receptor
regulator)
VN-0105 (JP)
(DPT-IPV/Hib vaccine)
VN-0107/MEDI3250 (JP)
(Nasal spray flu vaccine vaccine)
Intradermal Seasonal
Influenza Vaccine (JP)
(VN-100 /prefilled i.d. vaccine for
seasonal flu)
VN-101 (JP)
(Cell-culture H5N1 Influenza
vaccine)
U3-1565 (US/JP)
(Anti-HB-EGF antibody)
DS-8895 (JP)
(Anti-EPHA2 antibody)
DS-8273 (US)
(Anti-DR5 antibody)
DS-5573 (JP)
(Anti-B7-H3 antibody)
DS-8201 (JP)
(Anti-HER2 ADC)
23
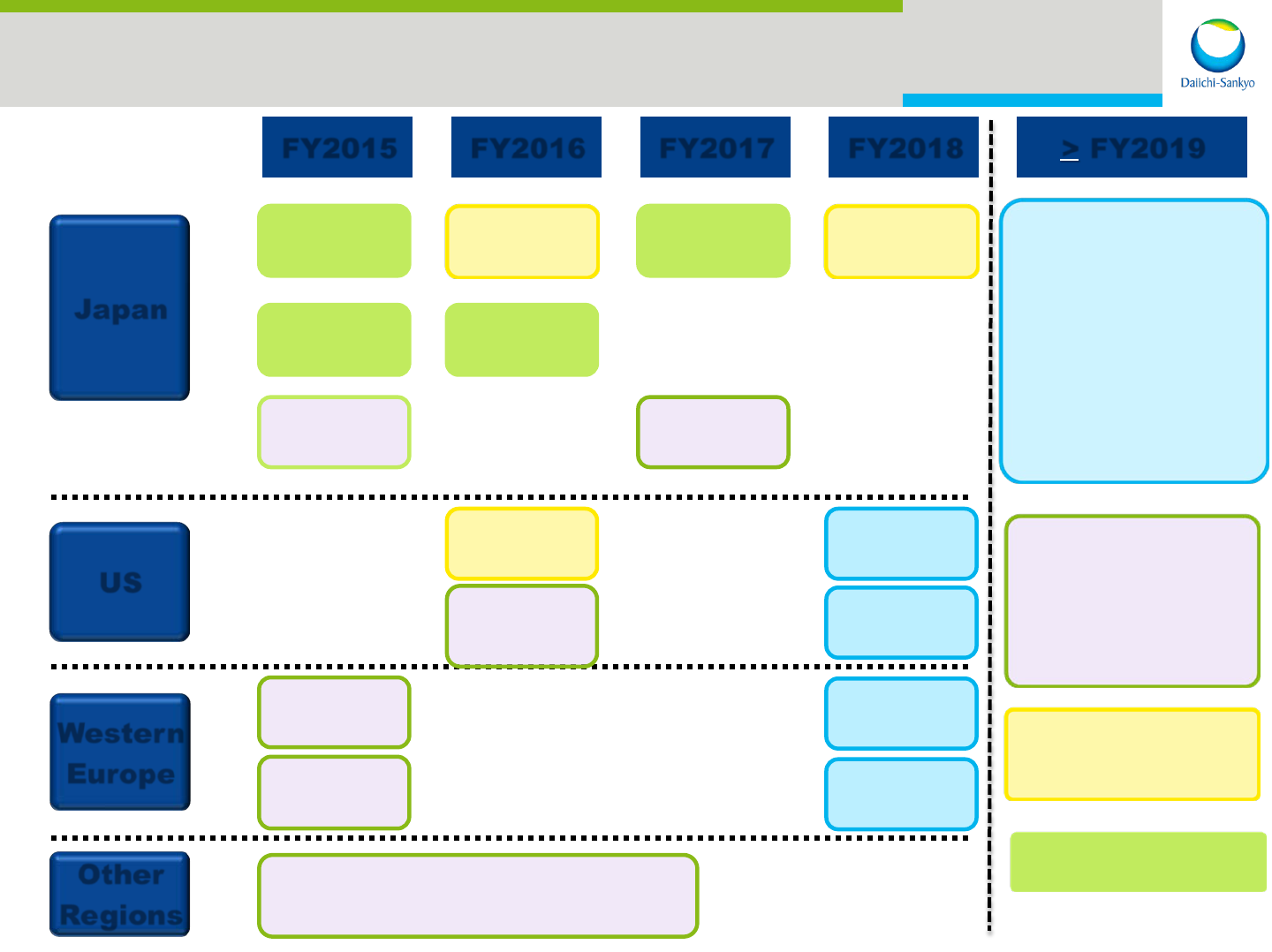
24
Japan
US
Western
Europe
Other
Regions
FY2015 FY2016 FY2017 FY2018
Lixiana
®
AF
Lixiana
®
VTE
Efient
®
CVA
Pralia
®
RA
Cravit
®
Injection
Lixiana
®
AF&VTE (China・LTAM etc.)
> FY2019
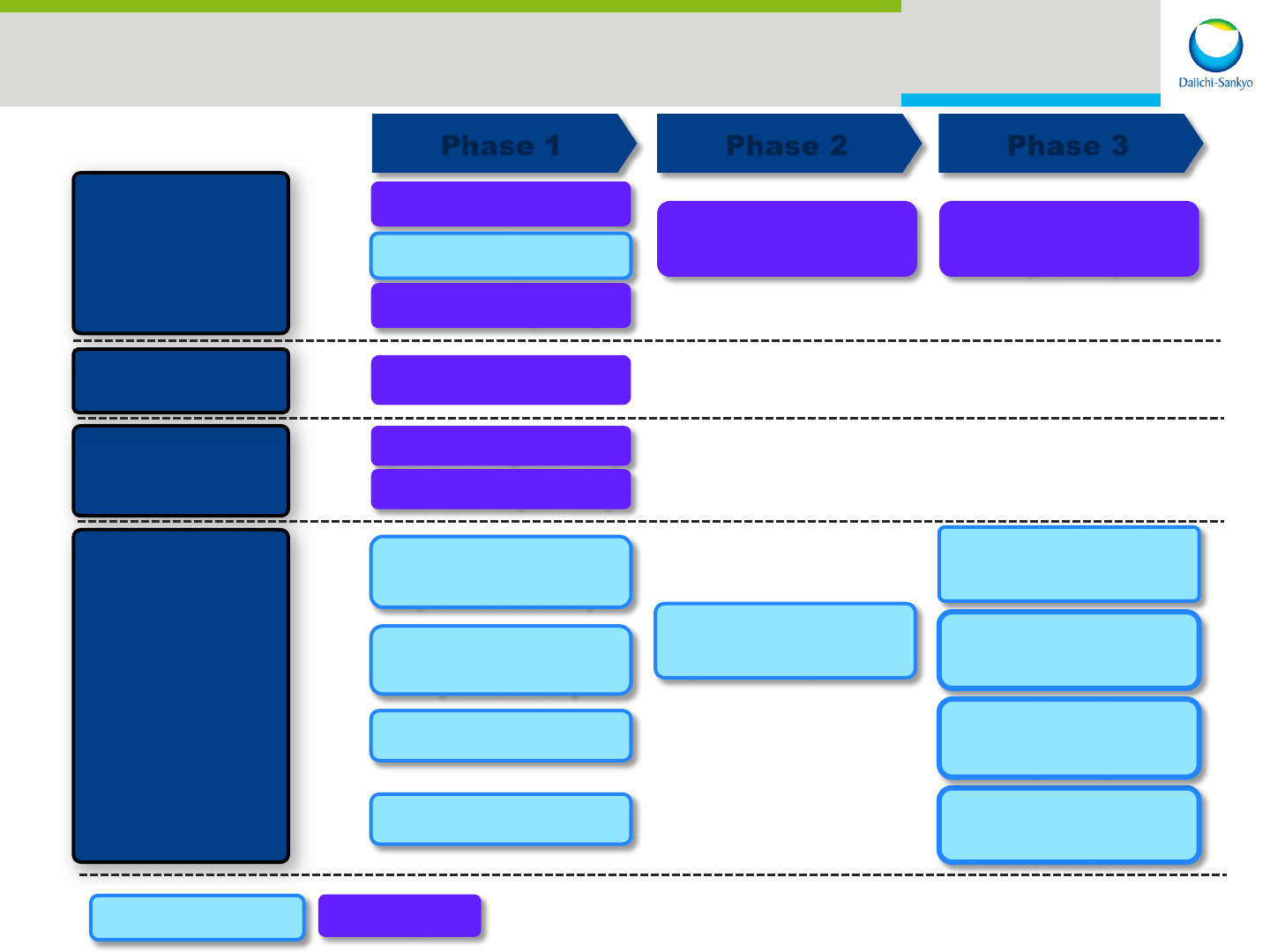
R&D: Targets for Approval and Launch
Oncology
Nimotuzumab
Patritumab
Pexidartinib
Quizartinib (JPN)
Zelboraf
®
(LCM)
Ranmark
®
(BC adj)
CV-M
CS-3150 (MRA)
DS-8500 (GPR119)
Effient
®
(LCM)
Lixiana
®
(LCM)
Pain
Mirogabalin
CL108
Acute Pain
& OINV
Hydromorphone
Cancer Pain
Lacosamide
Epilepsy
Tivantinib
HCC
Quizartinib
AML
Effient
®
Pediatric Exclusivity
Mirogabalin
DPNP & PHN
Tivantinib
HCC
Quizartinib
AML
Cravit
®
Tuberculosis
Artist
®
Chronic AF
Other
24

25
Daiichi Sankyo Oncology Strategy
25
Focus on first-in-class opportunities
Develop personalized medicine based therapies
Maintain strong academic partnerships
National Cancer Center of Japan
UCSF
Max Planck
Partner with innovative biotech companies
ArQule
Strategic acquisitions
Plexxikon
Ambit
26
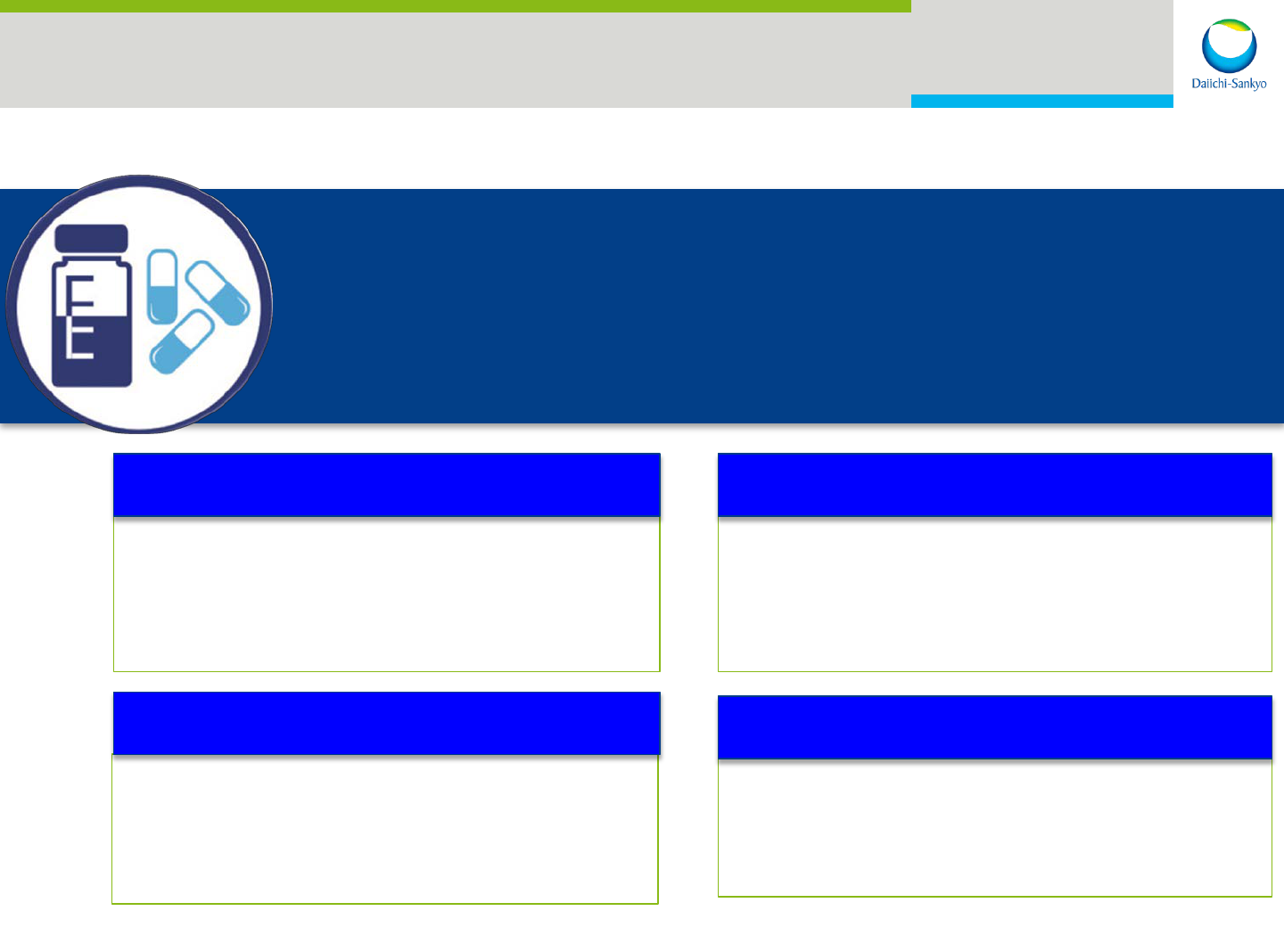
R&D: Oncology Clinical Pipeline
Growth
Survival
Receptors
U3-1565 (HB-EGF)
Patritumab (HER3)
Nimotuzumab
(EGFR)
Phase 1 Phase 2 Phase 3
DS-3032 (MDM2)
DS-8273 (DR5)
ADCC
DS-8895 (EPHA2)
DS-5573 (B7-H3)
ADC
Kinase
Pexidartinib
(FMS/KIT) GBM
Zelboraf
®
(BRAF)
MM Adjuvant
Tivantinib
(MET)
DS-6051
(NTRK/ROS1)
Pexidartinib
(FMS/KIT) TGCT
Quizartinib
(FLT3)
PLX7486
(FMS/TRK)
PLX8394 (mBRAF)
DS-8201 (HER2 ADC)
Small Molecule
Biologics
PLX9486 (mKIT)
ADC: Antibody Drug Conjugate
26
ADCC: Antibody Dependent Cellular Cytotoxicity
27
Late Stage Oncology Projects
Four novel compounds targeting
unique pathways in Phase 2/3
registration trials
• Acute myeloid leukemia (AML)
• TLR*: 1H 2017
Orphan Drug Designation by the FDA and EMA
Fast Track Status by the FDA
• Tenosynovial giant cell tumor (TGCT)
• TLR: 1H 2018
Orphan Drug Designation by the FDA and EMA
Breakthrough Therapy Designation by the FDA
• Hepatocellular carcinoma (HCC) in
partnership with ArQule
• TLR: 1H 2017
Orphan Drug Designation by the FDA and EMA
• Non-small cell lung cancer (NSCLC)
• TLR: 2H 2018
Quizartinib (Ph3) Pexidartinib (Ph3)
Tivantinib (Ph3)
Patritumab (Ph2/3)
*Projected top line results
27

28
R&D: Next Generation of DS Oncology Portfolio
Epigenetics
• IDH1 mutant inhibitor
• EZH 1/2 inhibitor
Immuno-oncology
• Immune checkpoint inhibitors
• Cell therapy
28

29
Collaboration with Merck:
• Pexidartinib in combination with anti-PD-1 therapy for
advanced melanoma and multiple other solid tumors
Other potential indications:
• Glioblastoma
• Ovarian cancer
• Breast cancer
• Sarcomas
Investigational CSF-1R (FMS) Inhibitor
• Tenosynovial Giant Cell Tumor (TGCT)
• Granted Orphan Drug Designation by the FDA and EMA
• Granted Breakthrough Therapy Designation by FDA
Pexidartinib: PLX3397
Further Investigations
29
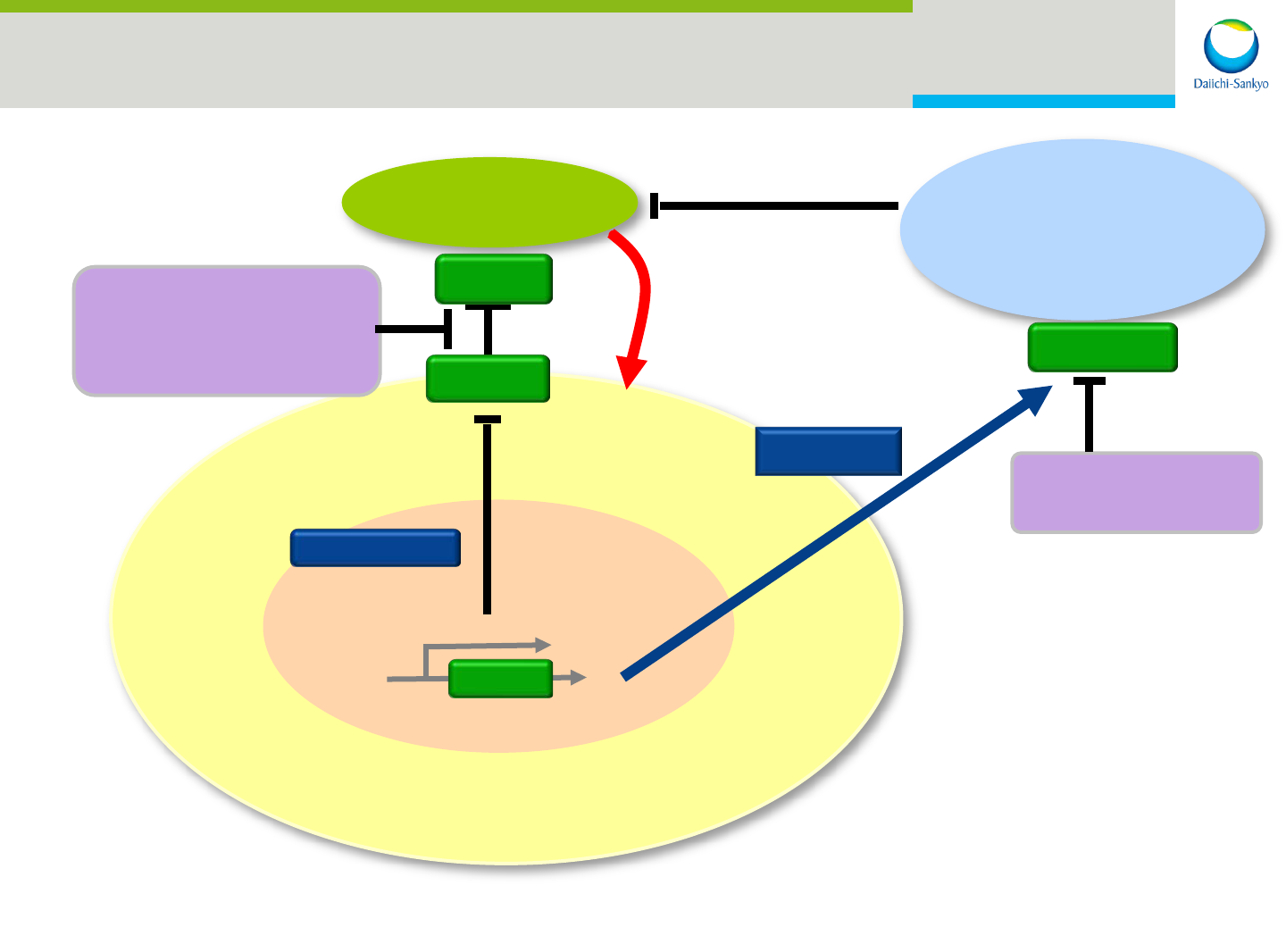
30
T CELL
Pexidartinib in Combination with anti-PD-1
Therapy for Advanced Solid Tumors
CANCER CELL
SIGNAL
PD-1
PD-L1
PD-1 mAb
Pembrolizumab
GENE
Myeloid
Suppressor
Cell
Pexidartinib
CSF1R
CSF1*
T-Cell-
Mediated
Killing
30
* colony stimulating factor 1

31
R&D: Next Generation of DS Oncology Portfolio
Epigenetics
• IDH1 mutant inhibitor
• EZH 1/2 inhibitor
Immuno-oncology
• Immune checkpoint inhibitors
• Cell therapy
31
32
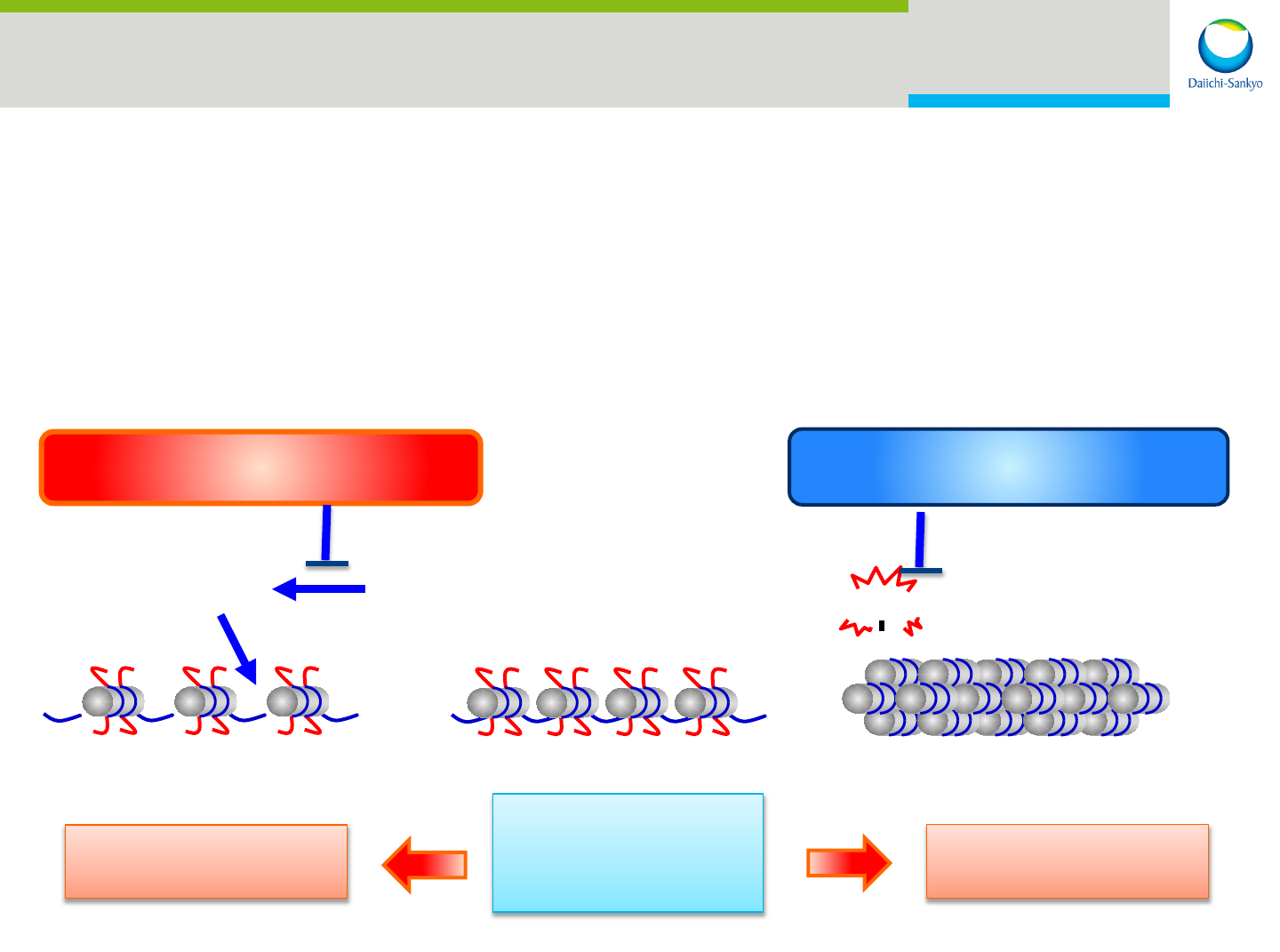
Epigenetic Targeting of Cancer Cells
• IDH1 mutant inhibitor decreases 2-hydroxyglutarate (2-HG) and improve
transcriptional abnormality
• EZH 1/2 inhibitor decreases histone methylation and increases transcription
of tumor suppressor genes
• Clinical studies of both inhibitors planned for 2016
Normal
cells
Cancer cells
Silencing of tumor suppressor genes
IDH1 mutant inhibitor
EZH1/2 dual inhibitor
2-HG
Histone
Methylation
H3K27
CH3
Transcriptional abnormality
Cancer cells
IDH1 mutant
32

Major Management Topics
Background
Edoxaban
Japan Business
US Business
R&D Topics (Oncology Strategy)
Conclusion
33
Today’s Agenda
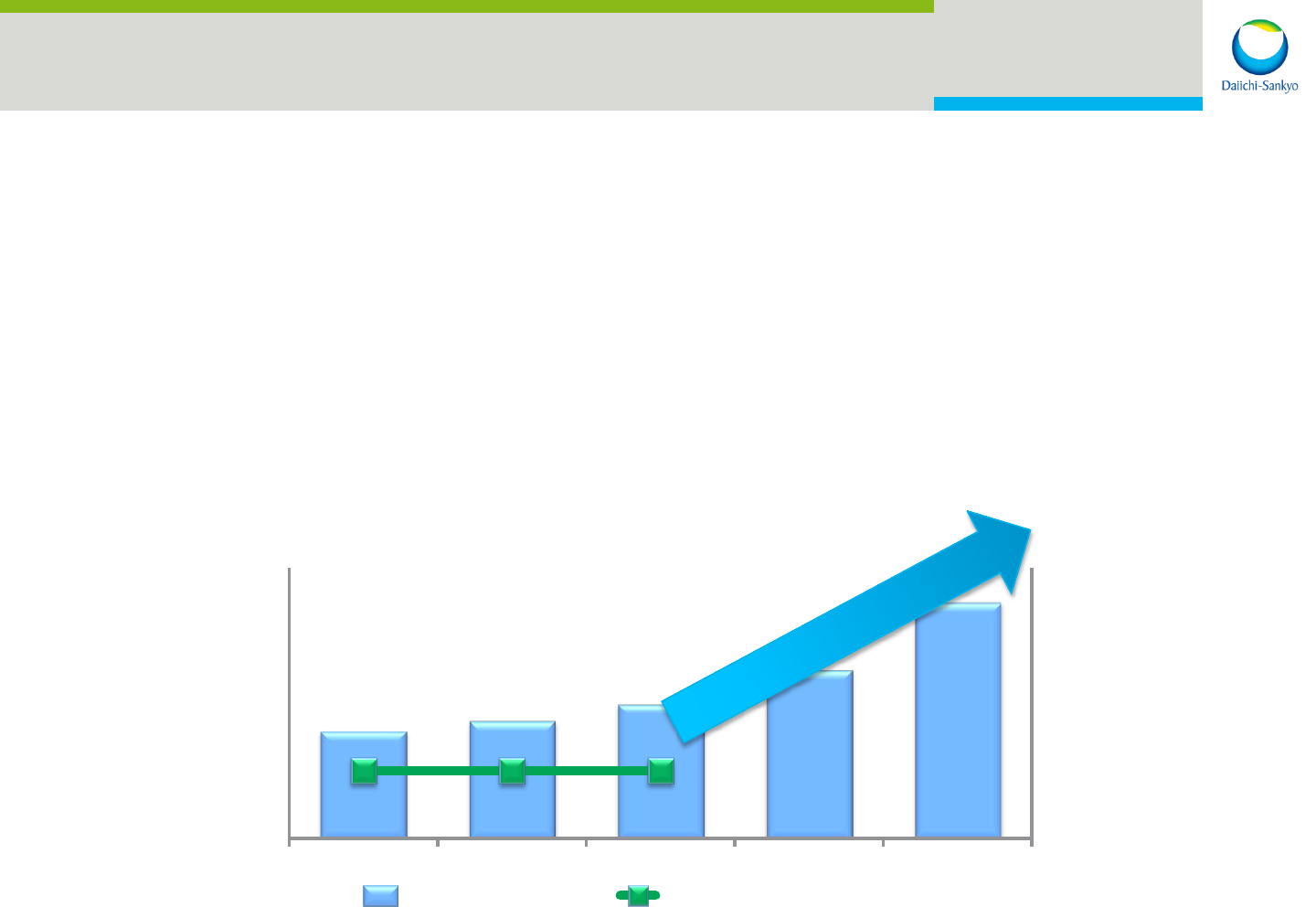
800
1000
1200
1400
1600
6000
8000
10000
12000
14000
FY15 FY16 FY17 FY18 ・・・・・・
(億円)
(億円)
Revenue Operating Profit
34
Next 5-year Business Plan
Period: FY2016 – FY2020
Contents:
Strategies for solid growth from FY2018
Improve profit-generating capability
Enhance R&D capabilities
Improve shareholder value (ROE) etc.
Announcement : March 2016
35
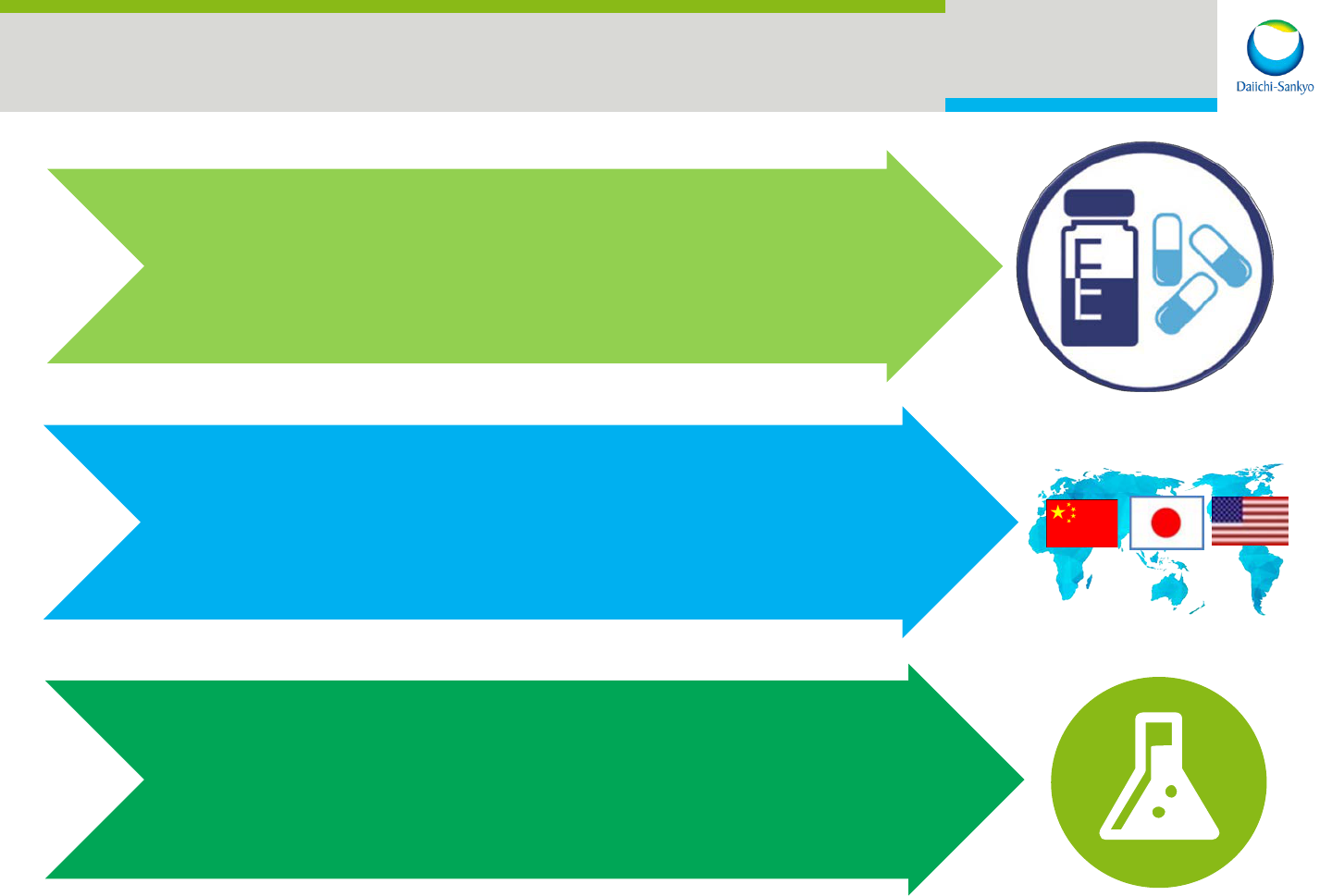
Business Priorities
Concentrate on
Innovative Business
Invest in Priority Regions:
Japan/US/China
Enhance R&D Capabilities
Core Business
Core Regions
Core Capabilities

Contact address regarding this material
Daiichi Sankyo Co., Ltd.
Corporate Communications Department
TEL: +81-3-6225-1126
Passion for Innovation.
Compassion for Patients.
TM
