
6 May 2015
GSK Investor Event

Sir Andrew Witty
Group overview and
strategic outlook
6 May 2015

>7 billion
people
Growing population…
>6 billion
people
outside US
& Europe
Driven by significant new cohorts…
~1 billion 60+
year olds
by 2020
(+20%)
650m
new
babies by
2020
Healthcare environment requires global, diversified and
innovative offering
Offset by sustained pricing pressure…
… and uncertainty of funding
Global
footprint
Broad portfolio
offering
Regulatory and
quality
competence
Science-led
innovation
3
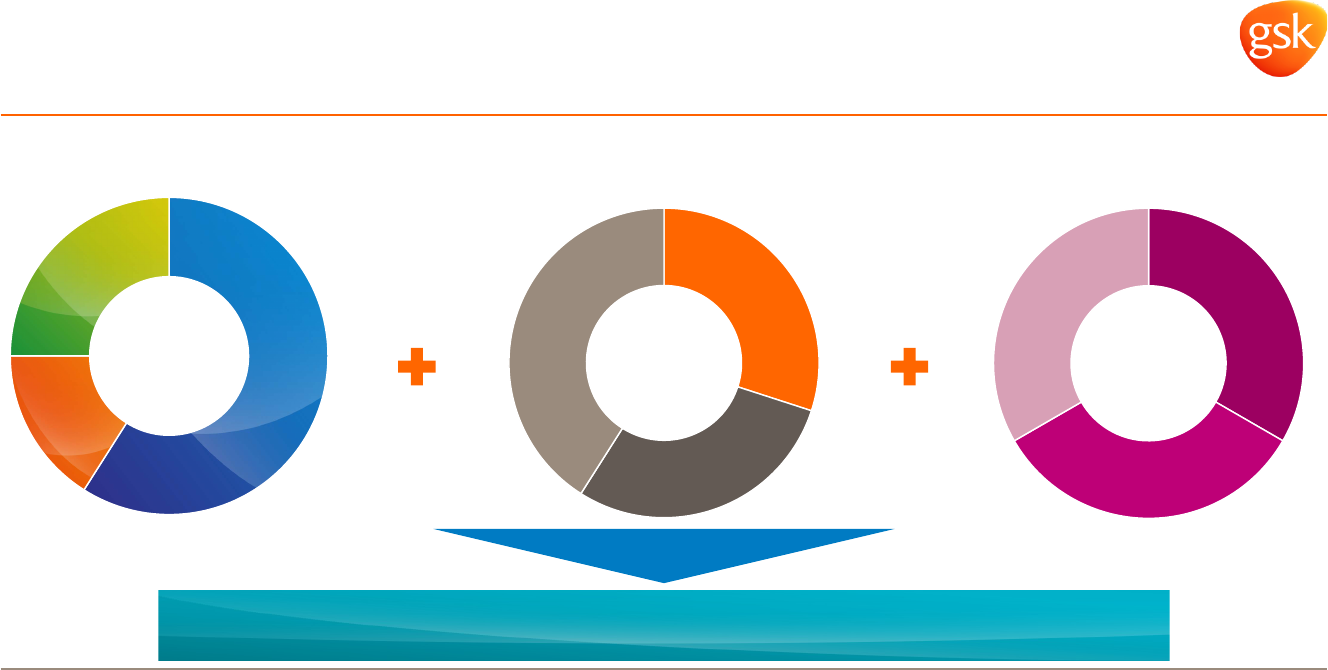
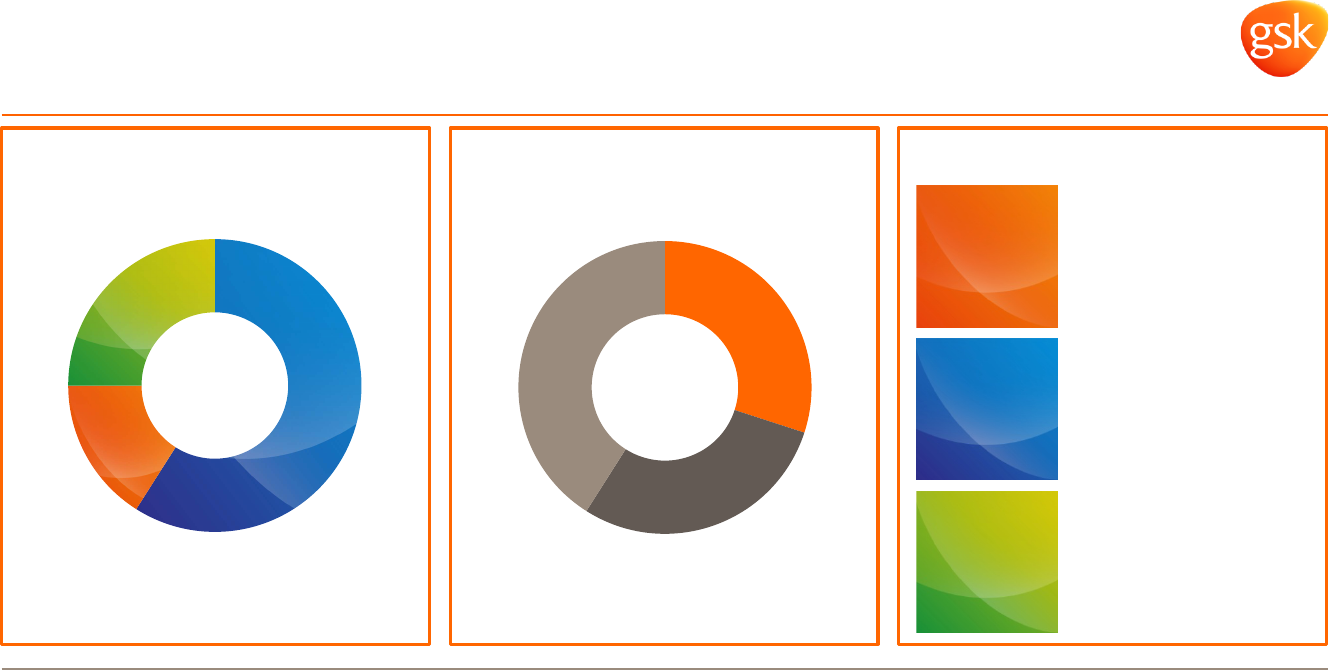
US
30%
EU
29%
Int
41%
Rx
59%
Vx
16%
Cx
25%
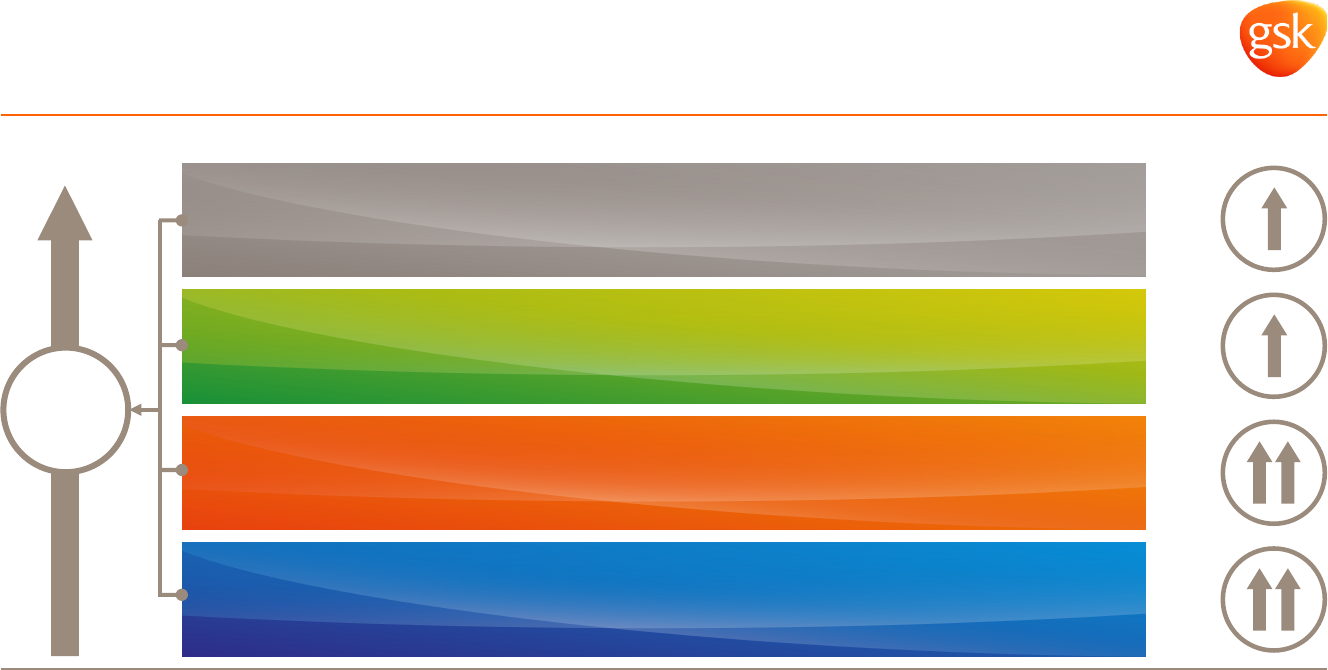
Long term strategic actions mean GSK is well positioned
for new operating environment
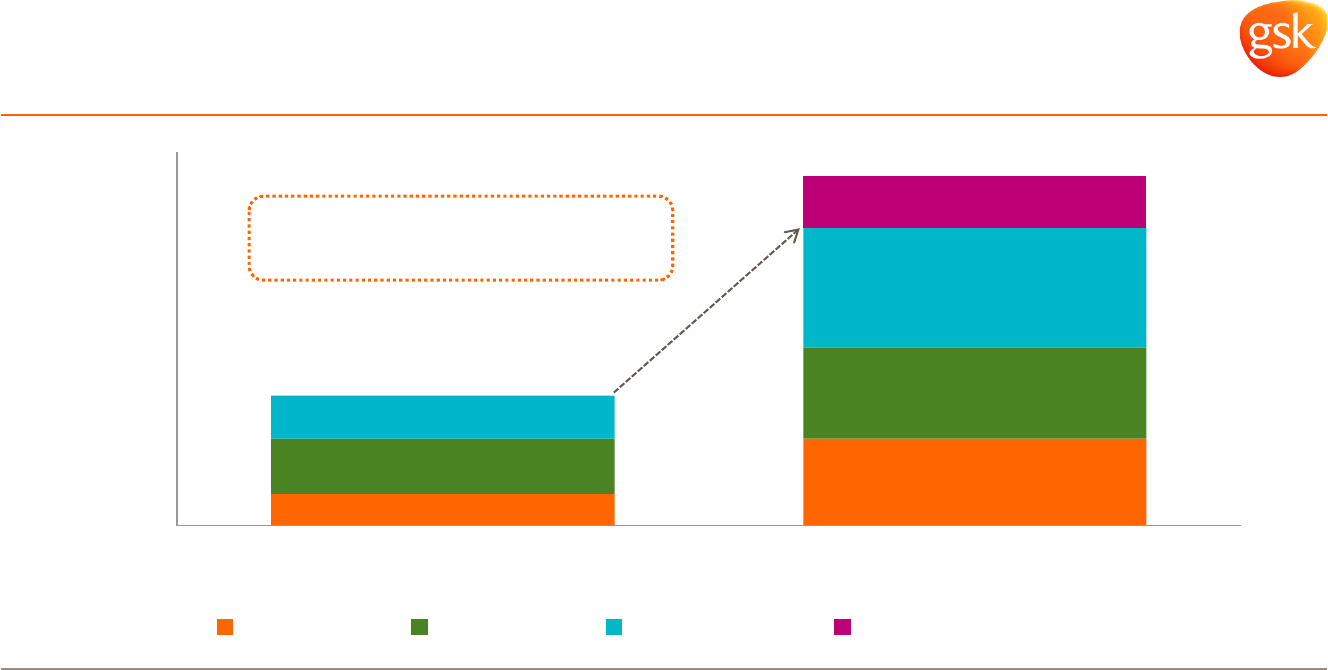
Diversified business* Diversified geographies* Cost saving programmes
£bn
Offering value for money health interventions to prevent and treat illness
Rx, 1.0
NVS,
1.0
Major
Change
1.0
* 2014 sales restated to exclude Oncology and include 12 months of NVS sales.
4
Capital allocation strategy to support growth
and returns
Investment
Flexibility
Shareholder return
Intention to retain full holding in ViiV
Accelerate restructuring of Group
Provide new flexibility for possible generic
Advair and ViiV/Consumer put options
3 year ordinary dividend of 80p 2015-2017
£1bn special dividend with Q4 2015
ordinary dividend
5

Rx
59%
Vx
16%
Cx
25%
Rx sales
Low single digit*
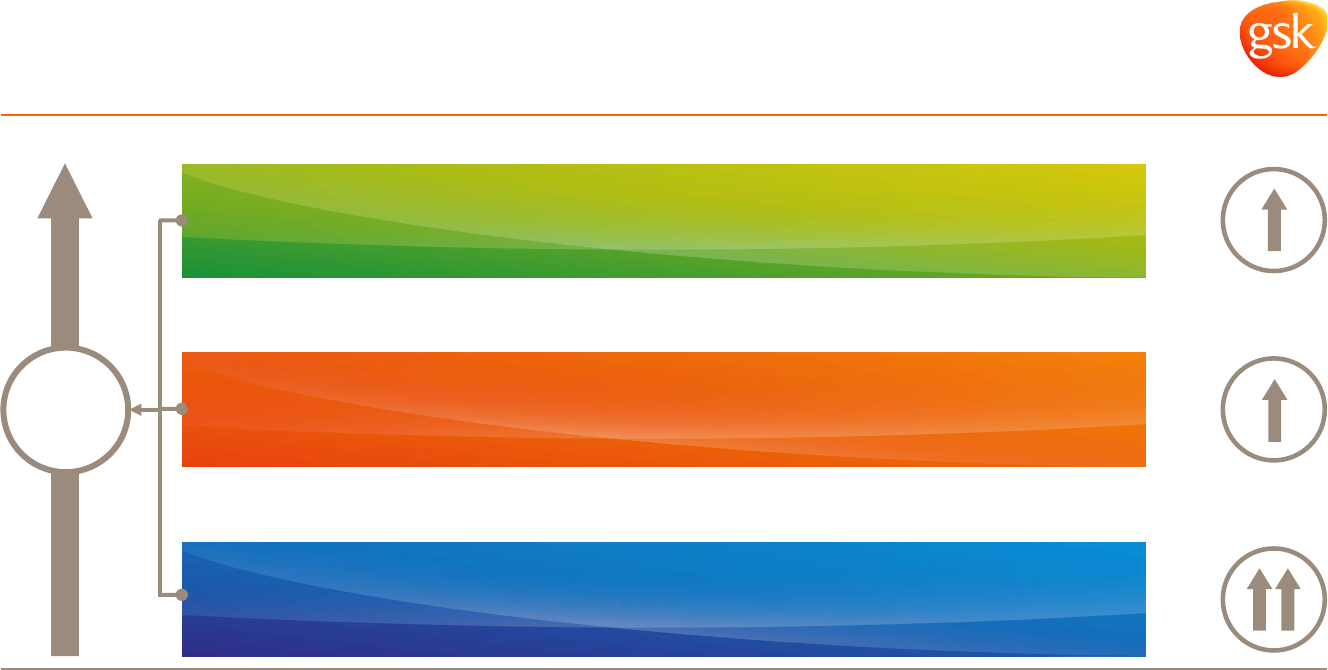
GSK targeting improvements to financial performance
2016-2020
Vx sales
Mid-to-high single digit*
Cx sales
Mid single digit*
Core EPS
2016 expected to
reach double digit CER growth
2016-2020 expected to be mid-
to-high single digit CER CAGR*
* CAGR to 2020, using 2015 as the base year. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and “Assumptions
and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015. All growth rates at CER.
Group sales
Expect low-to-mid single digit
CAGR for the Group*
12
month
2014 pro
forma**
** 2014 sales restated to exclude Oncology and include 12 months of NVS sales.
6
Key success factors
* Includes key recent and near-term launches plus late-stage assets. Rx: Breo, Anoro, Incruse, Arnuity, Tanzeum, Nucala, Tivicay, Triumeq, Vx: Menveo, Bexsero, Shingrix.
** Net PPE plus purchase of intangibles 2008-2014.
1
IMS
Cx
Accelerate growth with
strengthened portfolio
Geographic footprint expansion
Expand margins
Rx
New launches
Established products ex US/EU
Deliver pipeline
Vx
Expand coverage in USA
Improve reach in emerging markets
Expand margins
R&D
>£6bn sales from 11 new products by 2020
~40 Ph II/III NMEs
>30 DPUs
Commercial model
HCP
Sales force incentives
Digital
Quality/Supply
£11bn capital investments since 2008**
Volumes increased by 43% in emerging
markets since 2008
1
7
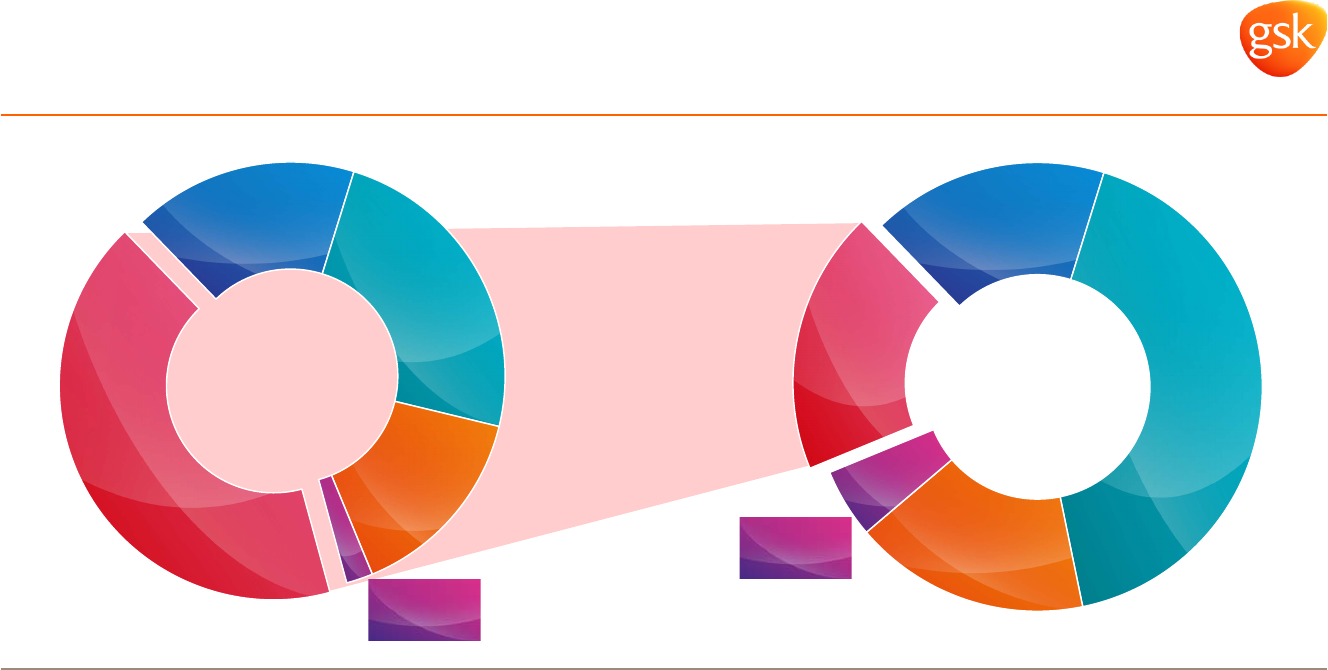
Broader and diversified portfolio offers sustained
revenue protection
* Advair, Flovent and Ventolin in the US, Europe and Japan.
** US, Europe and Japan.
*** All sales not captured by other categories.
2015 Q1
sales
2007
sales
Rx legacy
devices*,
17%
Cx & Vx,
24%
Rx outside
US/EU/Japan,
15%
Rx 10(+) year
patent**
2%
Other
Rx***,
42%
Rx legacy
devices*,
17%
Cx & Vx,
42%
Rx outside
US/EU/Japan,
17%
Rx 10(+) year
patent**
5%
Other
Rx***, 19%
8
High level exposure to
broad healthcare
markets and global
GDP growth
Volume driven
capability to drive
growth with reduced
reliance on price
Significant R&D
pipeline opportunities
and low concentration
of patent risk, post
Advair/Seretide
Leadership
positions in
Consumer &
Vaccines
Pharma
rebalanced
Expect sales and EPS
growth 2016-2020*
The GSK proposition
* CAGR to 2020, using 2015 as the base year. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and “Assumptions
and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015.
9

This presentation may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of
future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words
such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in
connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions,
prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the
outcome of contingencies such as legal proceedings, and financial results.
Other than in accordance with its legal or regulatory obligations (including under the UK Listing Rules and the Disclosure and Transparency
Rules of the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result
of new information, future events or otherwise. Investors should, however, consult any additional disclosures that the Group may make in any
documents which it publishes and/or files with the US Securities and Exchange Commission (SEC). All investors, wherever located, should
take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are cautioned
not to place undue reliance on the forward-looking statements.
Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the
Group’s control or precise estimate. The Group cautions investors that a number of important factors, including those in this document, could
cause actual results to differ materially from those expressed or implied in any forward-looking statement. Such factors include, but are not
limited to, those discussed under Item 3.D ‘Risk factors’ in the Group’s Annual Report on Form 20-F for 2014 and those discussed in Part 2 of
the Circular to Shareholders and Notice of General Meeting furnished to the SEC on Form 6-K on November 24, 2014. Any forward-looking
statements made by or on behalf of the Group speak only as of the date they are made and are based upon the knowledge and information
available to the Directors on the date of this report.
A number of adjusted measures are used to report the performance of our business. These measures are defined in our Q1 2015 earnings
release and annual report on Form 20-F.
Cautionary statement regarding forward-looking
statements

The unaudited pro forma financial information in this presentation has been prepared to illustrate the effect of (i) the disposal of the
oncology assets, (ii) the Consumer Healthcare joint venture (i.e. the acquisition of the Novartis OTC Business), and (iii) the acquisition of
the Vaccines business (which excludes the Influenza Vaccines business) on the results of the Group as if they had taken place as at
January 1, 2014.
The unaudited pro forma financial information has been prepared for illustrative purposes only and, by its nature, addresses a
hypothetical situation and, therefore, does not represent the Group’s actual financial position or results. The unaudited pro forma
financial does not purport to represent what the Group’s financial position actually would have been if the disposal of the Oncology
assets, the Consumer Healthcare joint venture and the Vaccines acquisition had been completed on the dates indicated; nor does it
purport to represent the financial condition at any future date.
In addition to the matters noted above, the unaudited pro forma financial information does not reflect the effect of anticipated synergies
and efficiencies associated with the Oncology disposal, the Consumer Healthcare joint venture and the Vaccines acquisition.
The unaudited pro forma financial information does not constitute financial statements within the meaning of Section 434 of the
Companies Act 2006. The unaudited pro forma financial information in this presentation should be read in conjunction with the financial
statements included in (i) the Group’s Q1 2015 earnings report dated May 6, 2015 and furnished to the SEC on Form 6-K, (ii) the
Group’s Annual Report on Form 20-F for 2014 and (iii) the Circular to Shareholders and Notice of General Meeting furnished to the SEC
on Form 6-K on November 24, 2014.
Unaudited pro forma financial information

Dr Moncef Slaoui
Vaccines
business overview
6 May 2015
The value of vaccination
Widely recognised as one of the very best investments in healthcare
UN, WHO, CDC.
13
…but still underserved populations
Target populations are growing…
…and major diseases remain without vaccines
~22m
infants still
missing basic
vaccines
~3m
deaths
prevented
annually
RSV
Group B Strep
TB
HIV
& more…
~1bn
60+ year olds
by 2020
(+20%)
Tremendous progress for global health…
Vaccines is an attractive business
• Growing market: ~£17bn in 2014
1
• Few global players
• Large capital investment
• Complex manufacturing
• Importance of combinations/lifecycle
management
• Intellectual property
• Very long product lifecycles
• Pharma like operating margins
14
1
Market data from Evaluate Pharma, GSK internal estimates.
#1 global company 2014 sales
GSK ~27%
pro forma
Pfizer
Merck
Sanofi
SPMSD
Novartis Vx
(ex. Flu)
2014 Annual Reports. Sales value for top 5 vaccine manufacturers (~80% of market).
GSK sales pro forma eliminating DT bulk sales.
Rixensart
Wavre
Gembloux
St Amand
Dresden
Godallo
Moscow
Ste-Foy
Philadelphia
Marietta
Hamilton
Laval
Bangalore
Nashik
Hyderabad
Shenzhen
Singapore
Shanghai
Rockville
Amsterdam
Limping
Ankleshwar
Siena/Rosia
Marburg
Cambridge
GSK Vaccines: a snapshot
* CapEx excludes Novartis investments. All other data represents pro forma business.
Arepandrix, Bexsero, Cervarix, Fendrix, Fluarix / FluLaval (QIV), Ixiaro, Menhibrix, Menitorix, Menveo, Pandemrix, Prepandrix, Priorix Tetra, Rotarix,
Synflorix. Excludes Nimenrix (to be divested).
**Includes major market approvals:
15
>2,000
scientists
~850m
doses in
2014
>16,000
people
14**
approvals
since
2005
7 R&D
sites
14 mfg
sites
~£4.2 bn*
capex
since
2005
0
500
1000
1500
2000
2500
3000
3500
4000
2005 2014
US sales EU sales ROW sales Novartis Portfolio (ex DT)
Strong track record of growth
Supply constraints impacted 2014 growth (-1% CER)
16
CAGR 2006-2014 ~8% CER
(ex Novartis)
CAGR 2006-2014 uses 2005 as base year.
Broadest vaccines portfolio offering worldwide (pre-transaction)
17
Key immunisation segments
Pediatric
Diphtheria, tetanus, & acellular Pertussis (DTaP)
DTaP hexa
Inactivated Polio (IPV)
Haemophilus influenzae type b (Hib)
Meningitis ACWY
Meningitis B
Pneumococcal
Measles, Mumps, Rubella (MMR) and Varicella
Rotavirus
P
Hepatitis A and B
Influenza
Adolescent
Human papillomavirus (HPV)
Tdap booster
Meningitis ACWY
Meningitis B
Hepatitis A and B
Influenza
Adults/Travellers
Tdap booster
YF
JE
TBE
Rabies
Typh
Hepatitis A and B
Influenza
Elderly
Zoster
P
Pneumococcal
Influenza
P – Project in late stage pipeline
Key immunisation segments US
Pediatric
Diphtheria, tetanus, & acellular Pertussis (DTaP)
DTaP hexa
Inactivated Polio (IPV)
Haemophilus influenzae type b (Hib)
Meningitis ACWY
Meningitis B
Pneumococcal
Measles, Mumps, Rubella (MMR) and Varicella
P
Rotavirus
P
Hepatitis A and B
Influenza
Adolescent
Human papillomavirus (HPV)
Tdap booster
Meningitis ACWY
Meningitis B
Hepatitis A and B
Influenza
Adults/Travellers
Tdap booster
YF
JE
TBE
Rabies
Typh
Hepatitis A and B
Influenza
Elderly
Zoster
P P
Pneumococcal
Influenza
Broadest vaccines portfolio offering worldwide (pre-transaction)
18
P – Project in late stage pipeline
World class, some volume constraints
Vaccines business
Supply
Recommendations
Portfolio breadth by segment
Geographic footprint
Price/Volume
R&D productivity and clinical trials
infrastructure
Keys to success GSK well-positioned
Strong ex-US, improving in US
>90% of portfolio with US/EU universal
recommendations
177 countries, global Rx benefits
Best in class mix
Over 1 million subjects in clinical trials
since 2000
Best in class (GSK estimate) Growth opportunities
19
Our strategic focus
Bolster
innovation
pipeline
Focus on
US approvals
and success
Reliable
sustainable
supply
Flawless
execution
Build
broader
talent pool
20

Our strategic focus
Bolster
innovation
pipeline
Focus on
US approvals
and success
Reliable
sustainable
supply
Flawless
execution
Build
broader
talent pool
Novartis transaction accelerates strategy
21
Strong portfolio synergy post-transaction
Key immunisation segments US
Pediatric
Diphtheria, tetanus, & acellular Pertussis (DTaP)
DTaP hexa
Inactivated Polio (IPV)
Haemophilus influenzae type b (Hib)
Meningitis ACWY
Meningitis B
Pneumococcal
Measles, Mumps, Rubella (MMR) and Varicella
P
Rotavirus
P
Hepatitis A and B
Influenza
Adolescent
Human papillomavirus (HPV)
Tdap booster
Meningitis ACWY
Meningitis B
Hepatitis A and B
Influenza
Adults/Travellers
Tdap booster
YF
JE
TBE
Rabies
Typh
Hepatitis A and B
Influenza
Elderly
Zoster
P P
Pneumococcal
Influenza
P – Project in late stage pipeline.
22
Key focus areas for 2015-2016
Manufacturing:
ongoing above
site, no disruption
within sites
Commercial
operations in
countries almost
complete
R&D:
accelerated and
portfolio review
completed
Delivery of cost
synergies:
~£400m by 2017
Novartis integration – well underway
Subject to “hold-separate” requirements of the vaccines businesses to be divested under EU commitments
23

Vaccines global R&D centre in US
Rockville, Maryland
24
Key focus areas for 2015-2016
25
Ensure
sustainability for
the long term
Designed to meet
and exceed
regulatory
requirements:
quality and
current GMP
Some supply
constraints
impacting HepA
and Pa containing
vaccines:
2014-2016
Proactive upgrading of supply network
State-of-the-art pertussis mfg site

Key growth drivers
Key near term drivers 2015-2016
Meningitis franchise, Flu QIV, Synflorix, Rotarix
26
Key growth drivers
Key near term drivers 2015-2016
Meningitis franchise, Flu QIV, Synflorix, Rotarix
New products 2017-2018
Expected launches: Shingrix (HZ/su), malaria, MMR US
Late stage development: Group B Strep, RSV, MenABCWY
27

• Risk of shingles doubles every decade over age 50
• Non-live, recombinant, 2-dose, adjuvanted vaccine
• Excellent efficacy across all age groups, ~97%
• Acceptable safety and tolerability
• Ongoing trials in 70+ and immuno-compromised
• Expect US, EU, Japan filings in 2016
• Low global penetration of current marketed vaccine
Shingrix HZ(su):
Significant opportunity to prevent herpes zoster
28
Key near term drivers 2015-2016
Meningitis franchise, Flu QIV, Synflorix, Rotarix
New products 2017-2018
Expected launches: Shingrix (HZ/su), malaria, MMR US
Late stage development: Group B Strep, RSV, MenABCWY
Key growth drivers
New segments 2019-2020 and beyond
Pregnant women
29
Key near term drivers 2015-2016
Meningitis franchise, Flu QIV, Synflorix, Rotarix
New products 2017-2018
Expected launches: Shingrix (HZ/su), malaria, MMR US
Late stage development: Group B Strep, RSV, MenABCWY
Key growth drivers
New segments 2019-2020 and beyond
Pregnant women
Mid-to-high
single
digit sales
growth*
Expected
CAGR 2016-20*
* Expected CAGR to 2020, using 2015 as the base year. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook”
and “Assumptions and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015.
All sales growth rates at CER.
30

Margin improvements
Improved leverage from sales growth
(CoGS, SG&A and disciplined R&D investments)
Transaction cost savings ~£400m by 2017
Maintain CapEx investments
GSK Vx (35.4%) + NVS loss making
~22% OPM 2014 pro forma
Overall vaccines margin 30%+ by 2020
All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and “Assumptions and cautionary statement regarding
forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015.
31

Positioned to be global leader for a very long time
Strong prospects for revenue and profit growth
Novartis transaction accelerates strategy
Bolster
innovation
pipeline
Focus on
US approvals
and success
Reliable
sustainable
supply
Flawless
execution
Build
broader
talent pool
All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and “Assumptions and cautionary statement regarding forward-looking
statements” sections of the Q1 Results Announcements dated 6 May 2015.
32

This presentation may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of
future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words
such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in
connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions,
prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the
outcome of contingencies such as legal proceedings, and financial results.
Other than in accordance with its legal or regulatory obligations (including under the UK Listing Rules and the Disclosure and Transparency
Rules of the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result
of new information, future events or otherwise. Investors should, however, consult any additional disclosures that the Group may make in any
documents which it publishes and/or files with the US Securities and Exchange Commission (SEC). All investors, wherever located, should
take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are cautioned
not to place undue reliance on the forward-looking statements.
Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the
Group’s control or precise estimate. The Group cautions investors that a number of important factors, including those in this document, could
cause actual results to differ materially from those expressed or implied in any forward-looking statement. Such factors include, but are not
limited to, those discussed under Item 3.D ‘Risk factors’ in the Group’s Annual Report on Form 20-F for 2014 and those discussed in Part 2 of
the Circular to Shareholders and Notice of General Meeting furnished to the SEC on Form 6-K on November 24, 2014. Any forward-looking
statements made by or on behalf of the Group speak only as of the date they are made and are based upon the knowledge and information
available to the Directors on the date of this report.
A number of adjusted measures are used to report the performance of our business. These measures are defined in our Q1 2015 earnings
release and annual report on Form 20-F.
Cautionary statement regarding forward-looking
statements

The unaudited pro forma financial information in this presentation has been prepared to illustrate the effect of (i) the disposal of the
oncology assets, (ii) the Consumer Healthcare joint venture (i.e. the acquisition of the Novartis OTC Business), and (iii) the acquisition of
the Vaccines business (which excludes the Influenza Vaccines business) on the results of the Group as if they had taken place as at
January 1, 2014.
The unaudited pro forma financial information has been prepared for illustrative purposes only and, by its nature, addresses a
hypothetical situation and, therefore, does not represent the Group’s actual financial position or results. The unaudited pro forma
financial does not purport to represent what the Group’s financial position actually would have been if the disposal of the Oncology
assets, the Consumer Healthcare joint venture and the Vaccines acquisition had been completed on the dates indicated; nor does it
purport to represent the financial condition at any future date.
In addition to the matters noted above, the unaudited pro forma financial information does not reflect the effect of anticipated synergies
and efficiencies associated with the Oncology disposal, the Consumer Healthcare joint venture and the Vaccines acquisition.
The unaudited pro forma financial information does not constitute financial statements within the meaning of Section 434 of the
Companies Act 2006. The unaudited pro forma financial information in this presentation should be read in conjunction with the financial
statements included in (i) the Group’s Q1 2015 earnings report dated May 6, 2015 and furnished to the SEC on Form 6-K, (ii) the
Group’s Annual Report on Form 20-F for 2014 and (iii) the Circular to Shareholders and Notice of General Meeting furnished to the SEC
on Form 6-K on November 24, 2014.
Unaudited pro forma financial information

Abbas Hussain
Pharmaceuticals
business overview
6 May 2015

Three commercial portfolios to drive revenue growth
Pharmaceuticals HIV
Sales organisation for ViiV*Sales and marketing of our
pure pharma business
Vaccines
In-country sales, marketing
and commercialisation of
vaccines portfolio
* In all markets excluding the 15 where ViiV has legal entities.
36

Changed the shape of our business
Successfully diversified our business to drive growth
and manage risk
Built a natural hedge in our portfolio
Internal financial data.
30 products
generating
sales of at
least £100m
25 markets
selling
£100m or
more
Total sales of pharmaceuticals & vaccines (% by geography)
2007
US
EM &
Others
HIV
Europe
US
EM &
Others
HIV
Europe
2014
40%
29%
24%
7%
31%
25%
36%
8%
37

Positioning us to succeed in a tough environment
Challenges
• Pricing
• Emerging Markets (EM) slowdown and
FX devaluations
Opportunities
• Demographics
• Respiratory access and pipeline
• ViiV expansion
• Broad vaccines portfolio
• Sustainable R&D
Lack of visibility
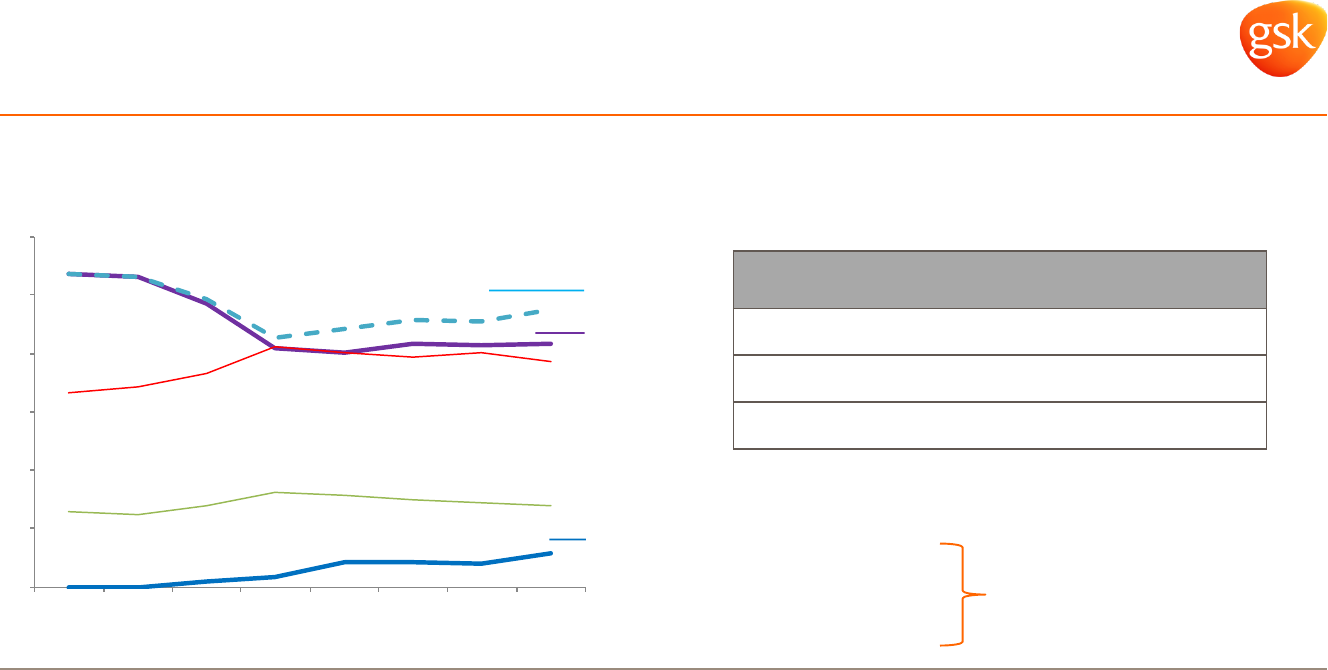
Advair US generics timing and impact
38
0%
10%
20%
30%
40%
50%
60%
Jun 13
Sep 13
Dec 13
Mar 14
Jun 14
Sep 14
Dec 14
Mar 15
Implemented multiple strategies to help
Seretide compete effectively outside the US
Advair access in US stabilised and back to
growth when combined with Breo
Pharmaceuticals: Respiratory
Proactively managing the decline of Seretide/Advair
Europe: Seretide pricing initiatives implemented
Emerging Markets: generics gained 2 volume share points in
24 months (in markets where a generic is present)
3
+10% value
+13% volume
Seretide 2-year total growth in
EMs where generic present
Advair
Advair+Breo
NBRx market share in US
1
Market
First generic
launch
Market share of
generic (Feb 15)
2
Germany June 2012 3.6%
Italy Sept 2013 1.0%
Netherlands Oct 2013 1.4%
1
IMS rolling weekly sales shown by quarter (March 2015).
2
IMS and other third party information.
3
IMS January 2015.
Competitor A
Competitor B
Breo
39
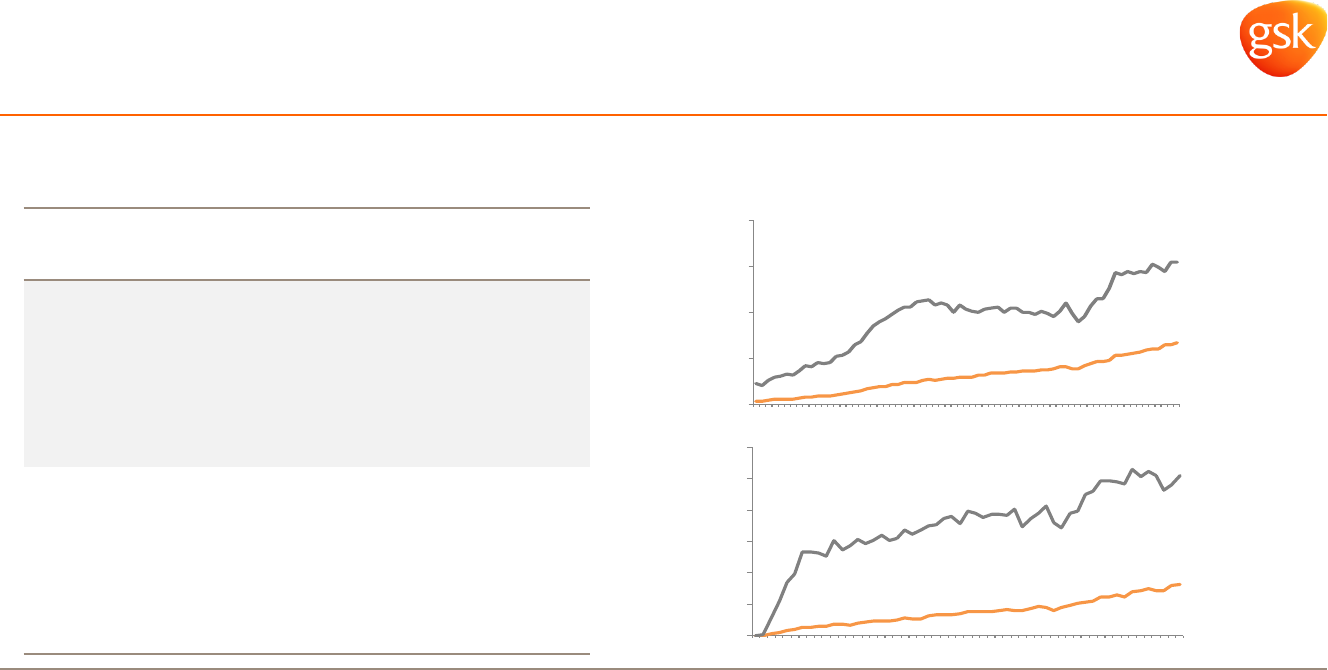
0%
2%
4%
6%
8%
10%
12%
W1
W3
W5
W7
W9
W11
W13
W15
W17
W19
W21
W23
W25
W27
W29
W31
W33
W35
W37
W39
W41
W43
W45
W47
W49
W51
W53
W55
0%
2%
4%
6%
8%
W1
W3
W5
W7
W9
W11
W13
W15
W17
W19
W21
W23
W25
W27
W29
W31
W33
W35
W37
W39
W41
W43
W45
W47
W49
W51
W53
W55
W57
W59
W61
W63
W65
W67
W69
Weekly uptake data improving as Breo and
Anoro share gains continue
2
Significant gains made in access over the
last 12 months
1
Pharmaceuticals: Respiratory
Strong US access for Breo and Anoro is driving uptake
Access
March
2014
March
2015
Commercial
Breo 49% 65%
Anoro 75% 83%
Medicare Part D
Breo 35% 74%
Anoro 0% 67%
1
MMIT March 2015.
2
IMS Weekly Data (as of 27 April 2015).
NBRx
TRx
NBRx
TRx
Breo market share in US
Anoro market share in US
40
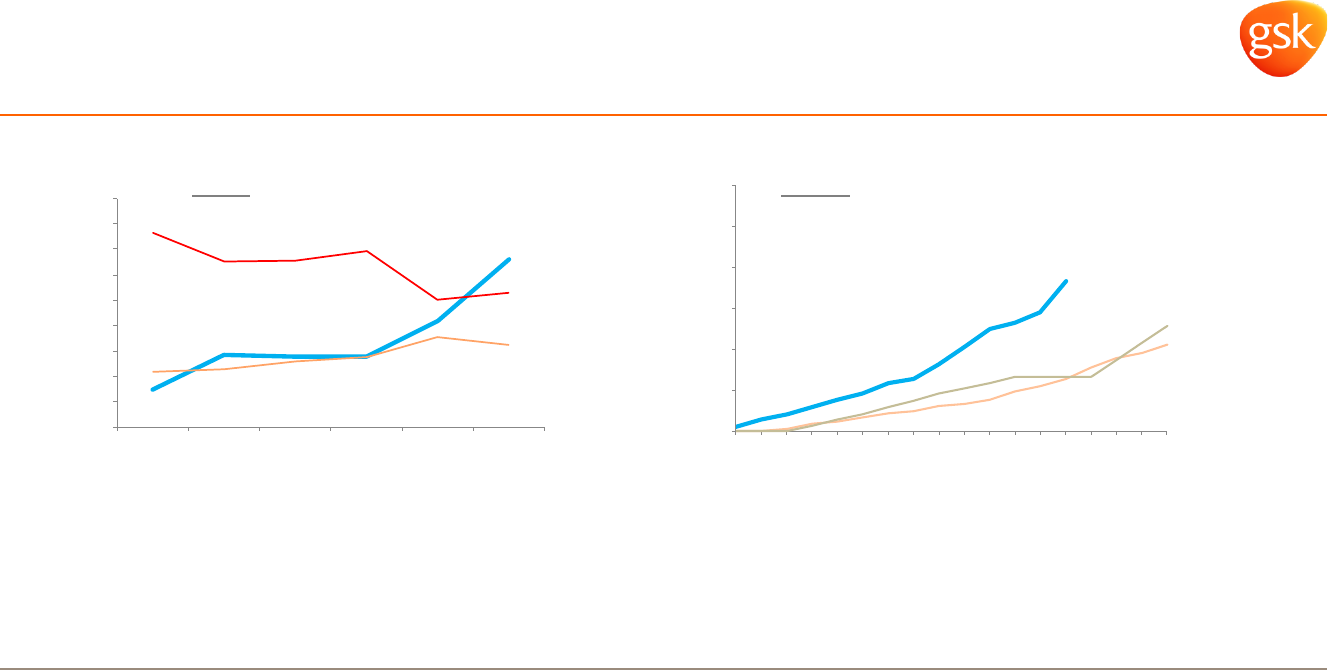
0%
5%
10%
15%
20%
25%
30%
35%
40%
45%
Dec13
Mar14
Jun14
Sep14
Dec14
Mar-15
0.0%
0.5%
1.0%
1.5%
2.0%
2.5%
3.0%
M1
M2
M3
M4
M5
M6
M7
M8
M9
M10
M11
M12
M13
M14
M15
M16
M17
M18
Competing well in key major markets...
Pharmaceuticals: Respiratory
Ex-US markets have good access, Relvar launching well
...with full launch potential still to be reflected
• Major European markets and Australia now have access
• Brazil and Mexico have launched; 16 EM launches planned to year-end
• SUMMIT data in 2H 2015 and Salford Lung study COPD data in 2H 2016 provide potential for upside
• Additional near-term pipeline (mepolizumab, closed triple) and Ellipta platform leverage
Japan: new patient share ICS/LABA
1
Relvar
Europe: market share ICS/LABA
2
Relvar
1
Rolling 3 month average (JMIRI G5 March 2015).
2
IMS
Competitor A
Competitor C
Competitor D
Competitor C
41
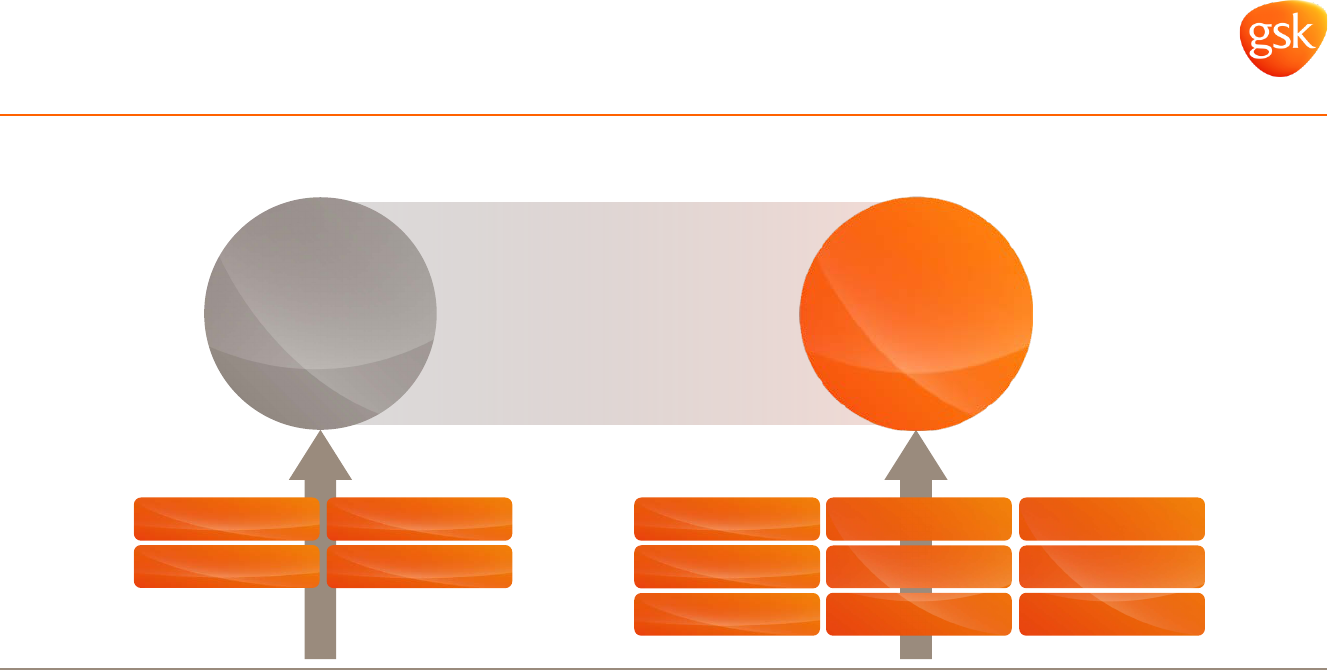
2020 expect total respiratory sales to be at or above sales in 2015, whether or not there is
US generic competition to Advair
Pharmaceuticals: Respiratory
Portfolio de-risked with balanced growth as new products gain scale
90% of 2015 sales
4
products
90% of 2020 sales
9
products
Avamys Seretide/Advair
Ventolin
Flixotide
Avamys
Relvar/Breo
Anoro
Incruse
mepolizumab
closed triple
Seretide/Advair
Flixotide
Ventolin
Internal financial data. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and “Assumptions
and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015.
42
Pharmaceuticals: Base Brands*
Generating volume and cash to support innovative brands
Applying commercial expertise to late-lifecycle management and access
Promote to Grow (60%): Drive volume post-
patent expiry through low cost promotion
Manage for Cash (40%): Rationalise tail
products and allocate based on margin
• Key assets growing low single digits outside US
• Centre of excellence in India
• Maximise existing supply
• Reduce complexities and simplify SKUs
• Targeted divestments
• Decreased SG&A
Antibacterials £789m -1%
Urology £805m +1%
Epilepsy £622m +5%
Removed over 4,500
SKUs; delivering
1% improvement
in gross margin
Internal financial data.
* Pharma ex-ViiV and ex-Respiratory.
43
0
2,000
4,000
6,000
8,000
10,000
12,000
14,000
16,000
Wk1
Wk10
Wk19
Wk28
Wk37
Wk46
Wk55
Wk64
Wk73
Wk82
TRx Volume
HIV
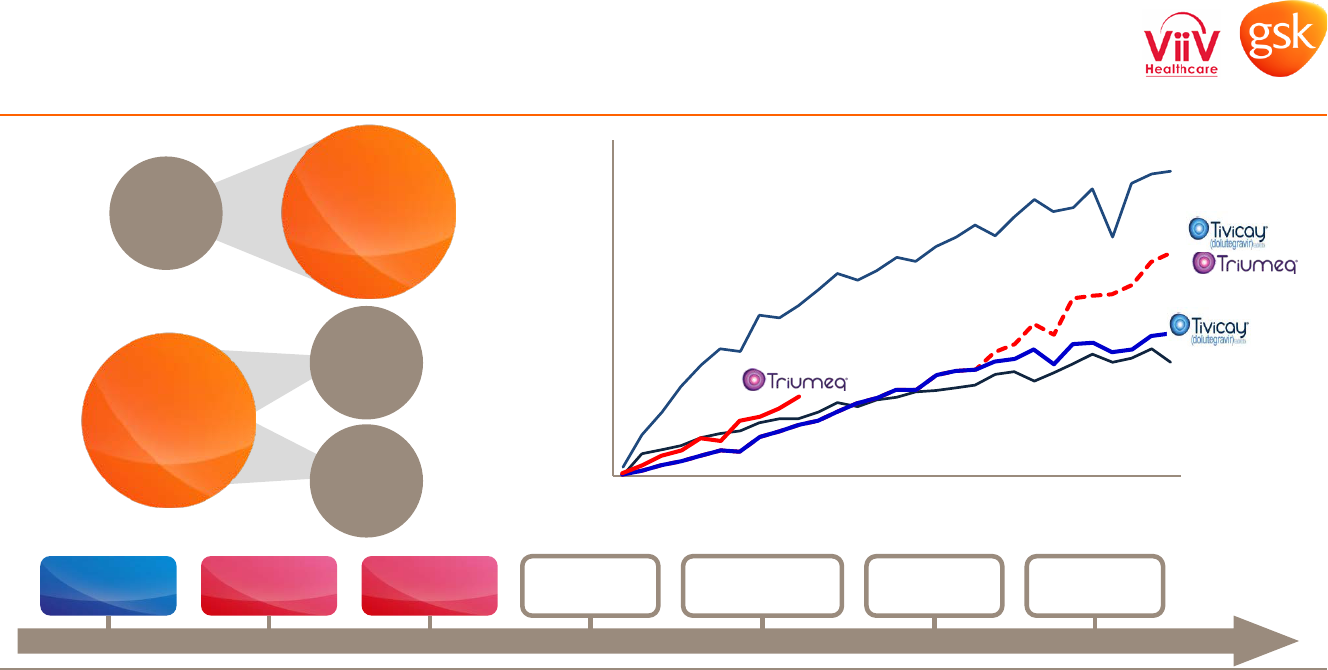
Rapidly growing business, transforming the market
Legacy
portfolio
Tivicay
DTG-based
regimens
cabotegravir
(‘744)
New
ARVs
Search for
cure
Triumeq
42%
growth in
Q1 2015
1
4%
growth in
Q1 2014
1
5
Tivicay
markets
2
Triumeq
markets
>90%
of total sales
1
US TRx 85 weeks post-Tivicay launch
2
1
Internal financial data.
2
IMS NPA Audit (4/3/15) and Symphony Health Solutions, CRx (3/27/15).
Competitor #1
Competitor #2
+
44

Vaccines
Balanced sources expected to drive growth from 2016-2020
Marketed Portfolio
• Driving top-line
synergies in Menveo
(US & International)
• Accelerating uptake
of Bexsero globally
• Successfully
launching
Shingrix
• Launching Mosquirix
in Africa
• Driving uptake in
unvaccinated
populations
• Sales synergies from
Novartis portfolio
Meningitis Portfolio Pipeline
1 2 3
45
2007
2014
2017
+12%
+23%
E
Sustainable pipeline flow in existing and new
growth areas
Pipeline and productivity
Strong future asset flow while restructuring drives margin
46
OpEx programmes are delivering improved
overall productivity
• Breo (asthma US decision, SUMMIT COPD)
• mepolizumab (severe asthma decision)
• sirukumab (RA PhIII data)
• ‘273 (ADA-SCID EMA filing)
• closed triple (COPD)
• cabotegravir (HIV)
• ‘863/PHI (anaemia)
• Shingrix (zoster vaccine)
• Respiratory (PI3Kδ)
• Inflammation (RIP kinases)
• Cardio-metabolic (TRPV4)
• Oncology (BETi, EZH2, LSD-1)
2015 Milestones
PhII/III Assets
Early Stage
1
Internal estimates. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and “Assumptions and cautionary statement
regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015.
Estimated sales/ sales FTE
Sales productivity (Rx+Vx+ViiV)
1
(est. sales per sales FTE)
Portfolio approach at market level gives flexibility to
deliver revenue growth
Strong operational management
Restructuring and Novartis synergies
Sustainable R&D pipeline
47
Expected
CAGR 2016-20*
Vaccines
Long-term growth creation with a strong perpetuity value
Mid-to
-high
single
digit*
Pharma (Respiratory)
Maintain topline and reduce dependency on Seretide/Advair
HIV
Immediate growth driver with untapped potential
Low
single
digit*
Pharma (Base Brands**)
Promote to Grow and Manage for Cash
**Pharma ex-ViiV and ex-Respiratory.
* Expected CAGR to 2020, using 2015 as the base year. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and
“Assumptions and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015. All sales growth rates at CER.

This presentation may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of
future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words
such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in
connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions,
prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the
outcome of contingencies such as legal proceedings, and financial results.
Other than in accordance with its legal or regulatory obligations (including under the UK Listing Rules and the Disclosure and Transparency
Rules of the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result
of new information, future events or otherwise. Investors should, however, consult any additional disclosures that the Group may make in any
documents which it publishes and/or files with the US Securities and Exchange Commission (SEC). All investors, wherever located, should
take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are cautioned
not to place undue reliance on the forward-looking statements.
Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the
Group’s control or precise estimate. The Group cautions investors that a number of important factors, including those in this document, could
cause actual results to differ materially from those expressed or implied in any forward-looking statement. Such factors include, but are not
limited to, those discussed under Item 3.D ‘Risk factors’ in the Group’s Annual Report on Form 20-F for 2014 and those discussed in Part 2 of
the Circular to Shareholders and Notice of General Meeting furnished to the SEC on Form 6-K on November 24, 2014. Any forward-looking
statements made by or on behalf of the Group speak only as of the date they are made and are based upon the knowledge and information
available to the Directors on the date of this report.
A number of adjusted measures are used to report the performance of our business. These measures are defined in our Q1 2015 earnings
release and annual report on Form 20-F.
Cautionary statement regarding forward-looking
statements

The unaudited pro forma financial information in this presentation has been prepared to illustrate the effect of (i) the disposal of the
oncology assets, (ii) the Consumer Healthcare joint venture (i.e. the acquisition of the Novartis OTC Business), and (iii) the acquisition of
the Vaccines business (which excludes the Influenza Vaccines business) on the results of the Group as if they had taken place as at
January 1, 2014.
The unaudited pro forma financial information has been prepared for illustrative purposes only and, by its nature, addresses a
hypothetical situation and, therefore, does not represent the Group’s actual financial position or results. The unaudited pro forma
financial does not purport to represent what the Group’s financial position actually would have been if the disposal of the Oncology
assets, the Consumer Healthcare joint venture and the Vaccines acquisition had been completed on the dates indicated; nor does it
purport to represent the financial condition at any future date.
In addition to the matters noted above, the unaudited pro forma financial information does not reflect the effect of anticipated synergies
and efficiencies associated with the Oncology disposal, the Consumer Healthcare joint venture and the Vaccines acquisition.
The unaudited pro forma financial information does not constitute financial statements within the meaning of Section 434 of the
Companies Act 2006. The unaudited pro forma financial information in this presentation should be read in conjunction with the financial
statements included in (i) the Group’s Q1 2015 earnings report dated May 6, 2015 and furnished to the SEC on Form 6-K, (ii) the
Group’s Annual Report on Form 20-F for 2014 and (iii) the Circular to Shareholders and Notice of General Meeting furnished to the SEC
on Form 6-K on November 24, 2014.
Unaudited pro forma financial information

Emma Walmsley
Consumer Healthcare
business overview
6 May 2015

The consumer healthcare opportunity
Increased
health
awareness
• Digital
• Consumer
• Retailer
Favourable
demographics
• Ageing population
• Emerging market
consumer
Growing
consumer
healthcare
market
• ~4% sales growth
1
• Healthy gross margin*
Pharma + FMCG
=
FMCH capabilities
1
Euromonitor. * Versus comparable businesses.
51

Our new portfolio strengthens category leadership positions
£6.1bn sales
1
1
12 month pro forma for 2014, including India & Nigeria.
2
Internal data.
3
Euromonitor.
4
Euromonitor - Total respiratory and smokers health.
5
Euromonitor, includes global
powdered drinks, sports nutrition, Vitamins.
Respiratory
£22bn
market
OTC / Wellness – 49%
2
Pain relief
£14bn
market
Gastro-
intestinal
£10bn
market
# 1
# 1
# 2
FMCG – 51%
2
Specialist
oral health
£5bn
market
Skin health
£8bn
market
# 1
# 1
India
# 3
Nutrition
£65bn
market
3
5
3
3
3
4
52
Competitive geographic footprint, sharper market focus
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
OTC
1
Specialist oral health
1
No. 1 OTC
worldwide
• No. 1 markets
from 13 to 36
Brands sold in
over 160
countries
• 42%
1
sales in
emerging markets
No. 1 specialist
oral health
worldwide
• No.1 in 50
markets
Leading pharmacy
coverage in China
Leader in OTC
in Germany
Nutrition brands in
over 1 million stores in
India
No. 1 toothpaste in
Turkey, 34% share
Top 3 OTC in world’s
largest healthcare
market in the US
Brands sold in over
60,000 pharmacies in
Russia
1
Internal data.
2
OTC, Euromonitor, Specialist Oral Care (Sensitivity, Denture Care, Gum Health, Dry mouth), IRI and Nielsen data via Compass.
2
2
53

7 Power Brands & 12 Core Brands will drive 90% of growth
7 Power Brands
12 Core Brands
Growth drivers
• Penetration opportunity
• Health care professional
recommendation
• Innovation
• Emerging markets
• Prioritised, high ROI
A&P
Theraflu includes carrier brands such as Beechams and Coldrex.
54

Track record of growth and innovation
• No. 1 dentist
recommended
• Strong innovation:
Repair & Protect /
Complete / True
White
• Trusted brand
• Consumer access
• Distribution strength
• Fastest growing OTC
brand
• Consumer insight: 12
hour claims and
packaging
+10%
CAGR
+12%
CAGR
+10%
CAGR
1
Euromonitor Data, retail sales 2005-2014.
1
1 1
55

Investing for long term innovation strength
• Consumer and science
led pipeline
• 6 co-located hubs, top
talent
• Sensory, packaging,
Shopper Science Lab
• Innovation >10% sales
1
1
Internal data.
56
Well placed to deliver sales growth
Expected
CAGR 2016-20*
Mid
single
digit*
Geographic footprint
Across 150 markets & sharper prioritisation
FMCH talent and capability
Consumer and science based innovation
Categories and brands
Global leadership & sharper prioritisation
* Expected CAGR to 2020, using 2015 as the base year. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and
“Assumptions and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015. All sales growth rates at CER.
57
Clear drivers for margin improvement
Power Brand focus
Supply chain improvement programme
SKU management, network consolidation
Transaction synergies: £400m by 2017
Headcount, procurement, standard processes
* Versus comparable businesses. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and “Assumptions and
cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015.
Target
20%+
margin*
(top quartile)
2020
58

A global consumer healthcare leader for the long term
Improved
prioritisation
and resource
allocation
Investing for
innovation
FMCH talent &
capabilities
Simplification &
cost reduction
Competitive
brand portfolio
and geographic
footprint
Integration accelerates strategy
Strong prospects for revenue and profit growth
All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and “Assumptions and cautionary statement regarding
forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015.
59

This presentation may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of
future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words
such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in
connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions,
prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the
outcome of contingencies such as legal proceedings, and financial results.
Other than in accordance with its legal or regulatory obligations (including under the UK Listing Rules and the Disclosure and Transparency
Rules of the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result
of new information, future events or otherwise. Investors should, however, consult any additional disclosures that the Group may make in any
documents which it publishes and/or files with the US Securities and Exchange Commission (SEC). All investors, wherever located, should
take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are cautioned
not to place undue reliance on the forward-looking statements.
Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the
Group’s control or precise estimate. The Group cautions investors that a number of important factors, including those in this document, could
cause actual results to differ materially from those expressed or implied in any forward-looking statement. Such factors include, but are not
limited to, those discussed under Item 3.D ‘Risk factors’ in the Group’s Annual Report on Form 20-F for 2014 and those discussed in Part 2 of
the Circular to Shareholders and Notice of General Meeting furnished to the SEC on Form 6-K on November 24, 2014. Any forward-looking
statements made by or on behalf of the Group speak only as of the date they are made and are based upon the knowledge and information
available to the Directors on the date of this report.
A number of adjusted measures are used to report the performance of our business. These measures are defined in our Q1 2015 earnings
release and annual report on Form 20-F.
Cautionary statement regarding forward-looking
statements

The unaudited pro forma financial information in this presentation has been prepared to illustrate the effect of (i) the disposal of the
oncology assets, (ii) the Consumer Healthcare joint venture (i.e. the acquisition of the Novartis OTC Business), and (iii) the acquisition of
the Vaccines business (which excludes the Influenza Vaccines business) on the results of the Group as if they had taken place as at
January 1, 2014.
The unaudited pro forma financial information has been prepared for illustrative purposes only and, by its nature, addresses a
hypothetical situation and, therefore, does not represent the Group’s actual financial position or results. The unaudited pro forma
financial does not purport to represent what the Group’s financial position actually would have been if the disposal of the Oncology
assets, the Consumer Healthcare joint venture and the Vaccines acquisition had been completed on the dates indicated; nor does it
purport to represent the financial condition at any future date.
In addition to the matters noted above, the unaudited pro forma financial information does not reflect the effect of anticipated synergies
and efficiencies associated with the Oncology disposal, the Consumer Healthcare joint venture and the Vaccines acquisition.
The unaudited pro forma financial information does not constitute financial statements within the meaning of Section 434 of the
Companies Act 2006. The unaudited pro forma financial information in this presentation should be read in conjunction with the financial
statements included in (i) the Group’s Q1 2015 earnings report dated May 6, 2015 and furnished to the SEC on Form 6-K, (ii) the
Group’s Annual Report on Form 20-F for 2014 and (iii) the Circular to Shareholders and Notice of General Meeting furnished to the SEC
on Form 6-K on November 24, 2014.
Unaudited pro forma financial information

Simon Dingemans
Financial outlook and
guidance
6 May 2015
Novartis transaction accelerates our strategy and
delivers against our financial objectives
Sales
growth
Operating
leverage
Financial
efficiency
Cash flow
growth
EPS
Free cash
flow
Better balanced and
broader range of
growth drivers
Significant synergy and
operating leverage
efficiencies
More balanced
and sustainable
cash flow
Sustained financial
efficiency
Returns to
shareholders
63
US
30%
EU
29%
Int
41%
Rx
59%
Vx
16%
Cx
25%
Better balanced and broader range of growth drivers
* 2014 sales restated to exclude Oncology and include 12 months of NVS sales.
** Includes key recent and near-term launches plus late-stage assets. Rx: Breo, Anoro, Incruse, Arnuity, Tanzeum, Nucala. Tivicay, Triumeq, Vx: Menveo, Bexsero, Shingrix.
Balanced segments* Balanced geographies* Balanced innovation**
8
New products
3
New products
10%+
Innovation
sales
Cx
Rx
Vx
64
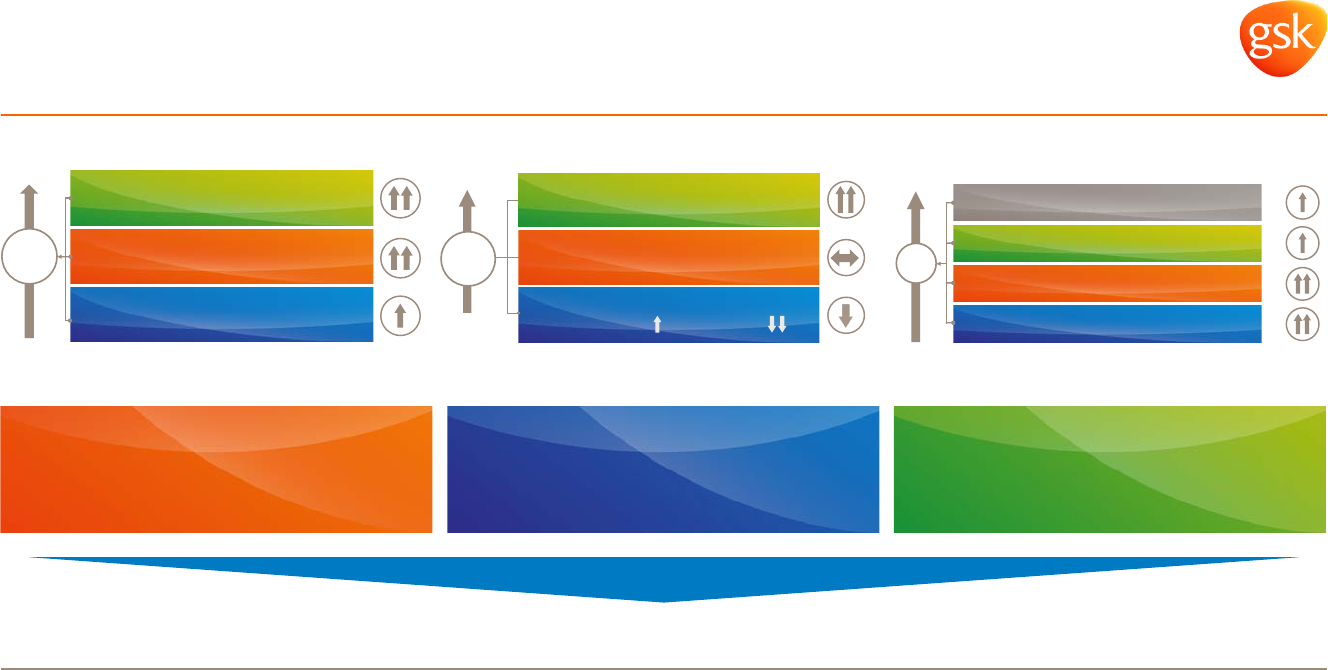
Delivering medium term sales growth
– 2016-2020 sales growth CAGR expectations*
Mid-to-high single digit Low single digit Mid single digit
– Group expectations: Low-to-mid single digit CAGR 2016-2020*
Vx Rx Cx
Key near term drivers 2015-2016
Meningitis franchise, Flu QIV, Synflorix, Rotarix
New products 2017-2018
Expected launches: Shingrix (HZ/su), malaria, MMR US
Late stage development: Group B Strep, RSV, MenABCWY
New segments 2019-2020 and beyond
Pregnant women
Mid-to-high
single
digit sales
growth**
Expected
CAGR 2016-20*
Expected
CAGR 2016-20
Mid
single
digit*
Geographic footprint
Across 150 markets & sharper prioritisation
FMCH talent and capability
Consumer and science based innovation
Categories and brands
Global leadership & sharper prioritisation
Pharma (Respiratory)
Maintain topline and reduce dependency on Seretide/Advair
HIV
Immediate growth driver with untapped potential
Low
single
digit*
Pharma (Base Brands**)
Promote to Grow and Manage for Cash
Expected
CAGR 2016-20
* Expected CAGR to 2020, using 2015 as the base year. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook”
and “Assumptions and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015. All growth rates at CER.
65
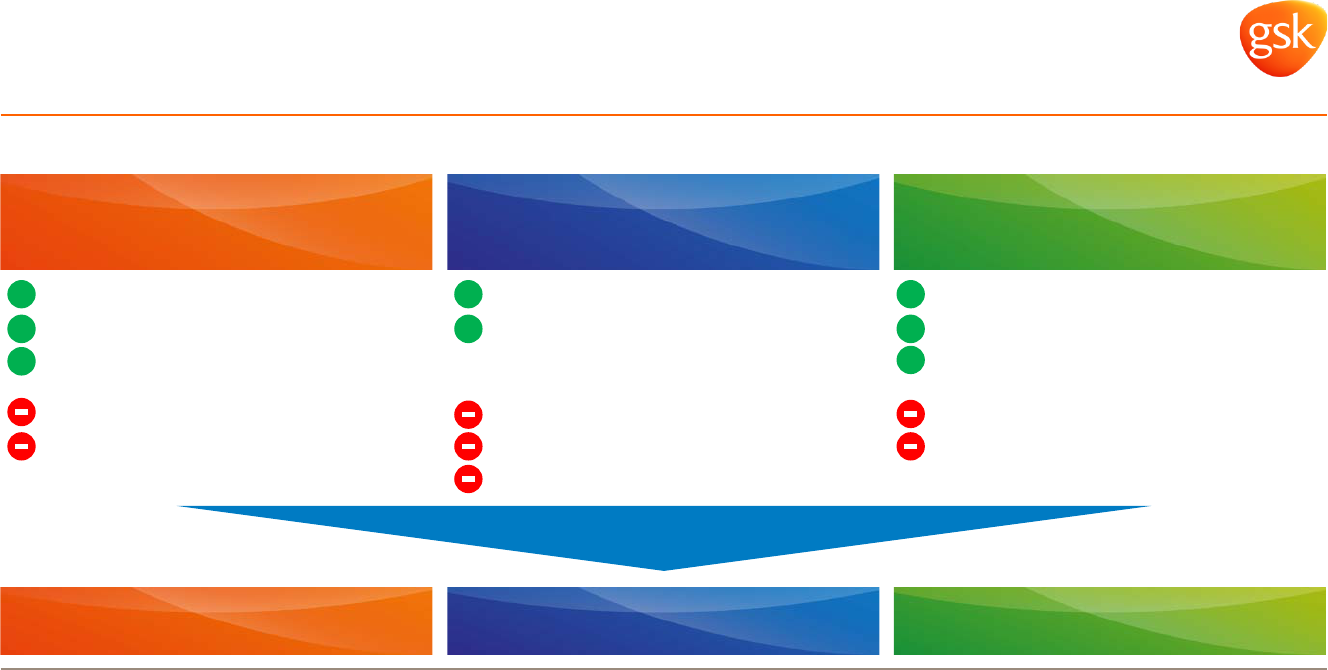
Enhanced operating leverage opportunities 2016-2020
Cost synergies
Revenue opportunities
Pipeline & new launches
New launches
Restructuring benefits
Cost synergies
Revenue opportunities
Supply chain improvement
Pipeline investment
Supply chain investment
US pricing
Product mix
R&D investment
Brand support
Innovation investment
+
+
+
+
+
+
* 2014 pro forma margin includes restatements to exclude Oncology and include 12 months of NVS business, as well as reallocation of corporate costs and after R&D.
30%+ Neutral vs 2015 20%+
Vx Rx Cx
11%
2014 Core OPM*
32%
2014 Core OPM*
22%
2014 Core OPM*
+
Targeting by 2020**
+
** CAGR to 2020, using 2015 as the base year. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and
“Assumptions and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015. All growth rates at CER.
66
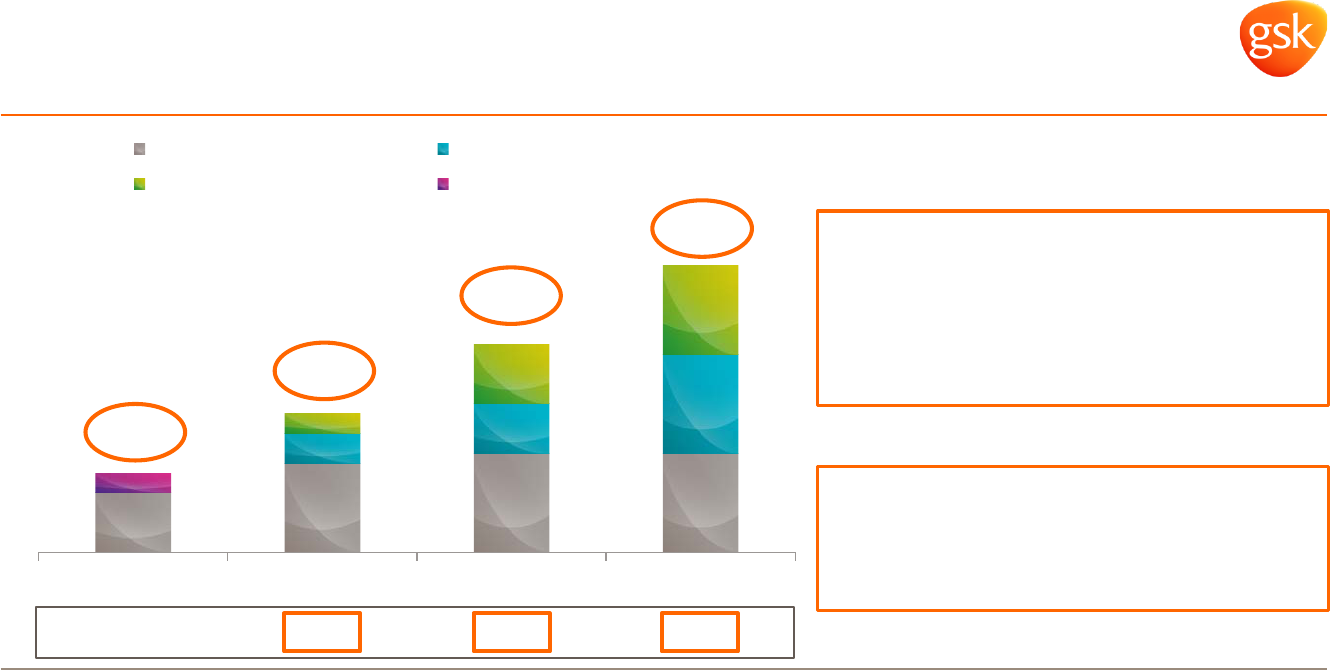
0.6
0.9
1.0 1.0
0.3
0.5
1.0
0.2
0.6
0.9
0.2
2014 2015 2016 2017
Major Change
Global Pharma restructuring
Novartis synergies Structural savings
0.8
2.9
2.1
1.4
Restructuring and structural savings
Total expected benefits from all three programmes ~£3bn
* Expected phasing of annual savings. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and “Assumptions and
cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015.
£bn*
Benefits delivery now accelerated
• Largely complete end 2017
• 20% reinvestment phased over ‘15-’17
Cash spend also accelerated in ’15-’16
Total costs of £5bn
• ~£3.65bn cash
• ~1.35bn non cash
Incremental saving
+0.6 +0.7 +0.8
Expensed to date
• £1.2bn cash
• £0.1bn non cash
67

Financial efficiency
Net finance costs
Sustained funding efficiency
Profits from associates
Not material post reduction of Aspen shares
Tax rate
No material change due to transaction:
Maintain expectation of 20% for 2015
Longer term subject to external environment
Minority interest
Step up reflecting Consumer and ViiV
Capital expenditure
Increased investment in 2015/16 - driving synergy & returns
68
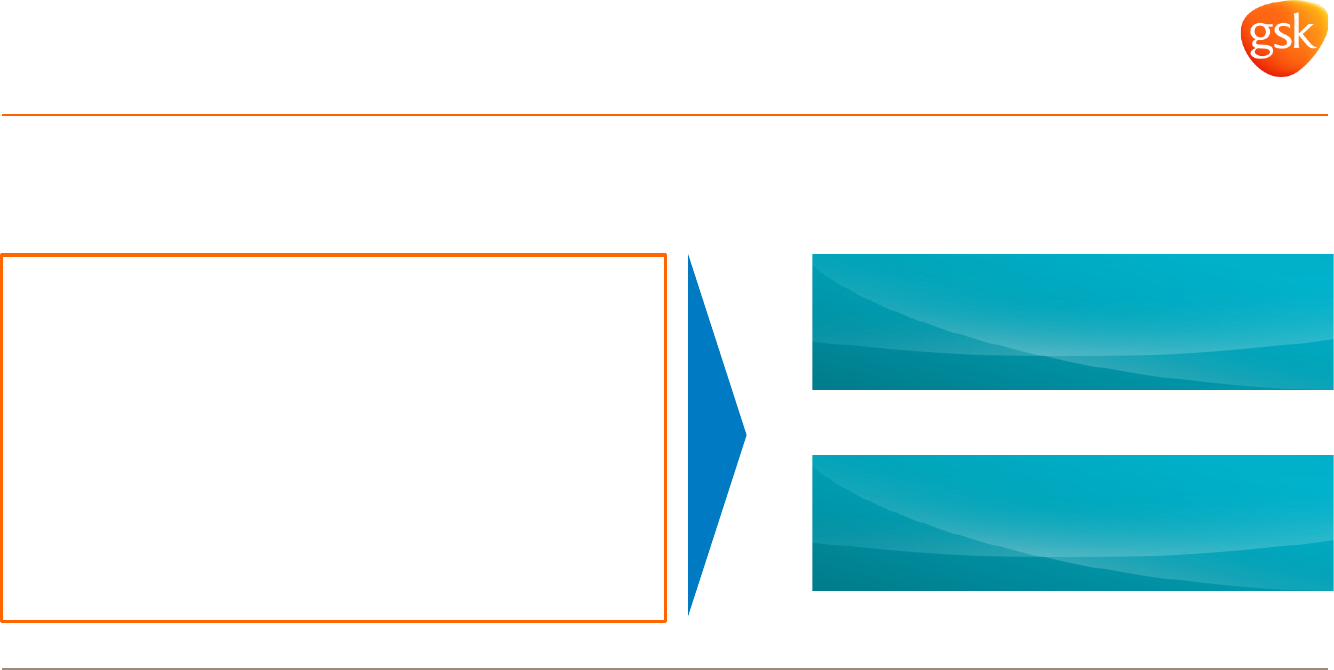
Capital allocation and shareholder returns
Maintain current credit ratings
Prioritise cash flows to
• Ordinary dividends
• Investment to accelerate synergies
Ensure flexibility to
• Respond to possible ViiV and Cx puts
• Absorb pressures of Gx Advair
Shareholder returns
80p dividend per share 2015-17
Special dividend Q4 2015
20p per share
Post transaction capital allocation
review completed
69
2015 guidance
Novartis transaction
Sales step up for Vaccines
and Consumer
Further decline in Rx
Respiratory transition,
inc US price & Oncology
exit offsetting new
launches & ViiV
Significant margin shift
Novartis impact, mix,
US Advair price &
structural savings
Royalties
Lower associates
Increased minority interest
Transaction impacts and
revised capital return
Expect high teens % decline in 2015 Core EPS (CER)*
* Compared to 95.4p core GSK reported 2014 EPS. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook” and
“Assumptions and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015.
70

NVS transaction impacts
~ -6% to -8%
Impact on 2015 EPS growth*
Regulatory divestments
Revised capital return
Synergy phasing
Inherited cost base
* All growth rates CER. 2015 growth is compared to 95.4p core GSK reported 2014 EPS. All expectations and targets regarding future performance should be read together with
the “2015-2020 Outlook” and “Assumptions and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015.
2015 Core EPS: Expected decline of high teens % (CER)*
2016 Core EPS: Expected to reach double digit % growth (CER)
71
GSK reshaped: Delivering on our strategy and financial
architecture
Low to mid
single digit
sales growth
Improved operating
leverage
Sustained financial
efficiency
More balanced and
sustainable cash flow
Core EPS expectations
2016-2020 mid-to-high
single digit growth CER*
Returns to shareholders
Plan to pay annual ordinary
dividend of 80p per share
2015-2017
Special dividend of 20p
with Q4 2015 dividend
Vx sales
Mid-to-high single digit
growth*
Rx sales
Low single digit
growth*
Cx sales
Mid single digit
growth*
* Expected CAGR to 2020, using 2015 as the base year. All expectations and targets regarding future performance should be read together with the “2015-2020 Outlook”
and “Assumptions and cautionary statement regarding forward-looking statements” sections of the Q1 Results Announcements dated 6 May 2015. All growth rates at CER.
72

This presentation may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of
future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words
such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in
connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions,
prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the
outcome of contingencies such as legal proceedings, and financial results.
Other than in accordance with its legal or regulatory obligations (including under the UK Listing Rules and the Disclosure and Transparency
Rules of the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result
of new information, future events or otherwise. Investors should, however, consult any additional disclosures that the Group may make in any
documents which it publishes and/or files with the US Securities and Exchange Commission (SEC). All investors, wherever located, should
take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are cautioned
not to place undue reliance on the forward-looking statements.
Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the
Group’s control or precise estimate. The Group cautions investors that a number of important factors, including those in this document, could
cause actual results to differ materially from those expressed or implied in any forward-looking statement. Such factors include, but are not
limited to, those discussed under Item 3.D ‘Risk factors’ in the Group’s Annual Report on Form 20-F for 2014 and those discussed in Part 2 of
the Circular to Shareholders and Notice of General Meeting furnished to the SEC on Form 6-K on November 24, 2014. Any forward-looking
statements made by or on behalf of the Group speak only as of the date they are made and are based upon the knowledge and information
available to the Directors on the date of this report.
A number of adjusted measures are used to report the performance of our business. These measures are defined in our Q1 2015 earnings
release and annual report on Form 20-F.
Cautionary statement regarding forward-looking
statements

The unaudited pro forma financial information in this presentation has been prepared to illustrate the effect of (i) the disposal of the
oncology assets, (ii) the Consumer Healthcare joint venture (i.e. the acquisition of the Novartis OTC Business), and (iii) the acquisition of
the Vaccines business (which excludes the Influenza Vaccines business) on the results of the Group as if they had taken place as at
January 1, 2014.
The unaudited pro forma financial information has been prepared for illustrative purposes only and, by its nature, addresses a
hypothetical situation and, therefore, does not represent the Group’s actual financial position or results. The unaudited pro forma
financial does not purport to represent what the Group’s financial position actually would have been if the disposal of the Oncology
assets, the Consumer Healthcare joint venture and the Vaccines acquisition had been completed on the dates indicated; nor does it
purport to represent the financial condition at any future date.
In addition to the matters noted above, the unaudited pro forma financial information does not reflect the effect of anticipated synergies
and efficiencies associated with the Oncology disposal, the Consumer Healthcare joint venture and the Vaccines acquisition.
The unaudited pro forma financial information does not constitute financial statements within the meaning of Section 434 of the
Companies Act 2006. The unaudited pro forma financial information in this presentation should be read in conjunction with the financial
statements included in (i) the Group’s Q1 2015 earnings report dated May 6, 2015 and furnished to the SEC on Form 6-K, (ii) the
Group’s Annual Report on Form 20-F for 2014 and (iii) the Circular to Shareholders and Notice of General Meeting furnished to the SEC
on Form 6-K on November 24, 2014.
Unaudited pro forma financial information

Appendix

Turnover
Operating Profit
Operating Margin
Total Pharma
14.3
4.5
31.7%
Vaccines
3.7
0.8
22.4%
Consumer
6.1
0.7
11.0%
Corporate
0.1
0.1
***
Total 12 month* pro forma
24.2
6.1
25.2%
£bn at 2014 actual rates
12 month* pro forma 2014
The major adjustments to sales and operating profit to calculate the restated figures above are:
- exclude Oncology**;
- include 12 months of the acquired Novartis Consumer and Vaccines businesses;
- reallocate most corporate costs to more accurately reflect the profitability of each segment; and
- reallocate divestments required to Corporate.
* 12 month pro forma provided for modelling purposes. The pro forma growth rates provided in the quarterly results adjust from March onwards, as explained within the Q1 press release.
**Oncology comprises the Company’s Marketed Oncology Portfolio, related R&D activities and rights to its AKT Inhibitors currently in development and also the grant to Novartis of the
Oncology Commercialisation Partner Rights for future oncology products arising from GSK’s early-stage oncology pipeline.
*** Corporate operating profit includes a structural benefit of £219m that was realised in Q3 2014.
76
Currency
US $
10 cents movement in average exchange rate for full year
impacts EPS by approx. +/- 3%
Euro €
10 cents movement in average exchange rate for full year
impacts EPS by approx. +/- 2%
Japanese ¥
10 Yen movement in average exchange rate for full year
impacts EPS by approx. +/- 1%
US $ 32
%
Euro € 20
%
Japanese ¥ 7
%
Other* 41
%
Period end exchange rates for March 2015 were £1/$1.48, £1/€1.38 and £1/Yen 178
If exchange rates were to hold at the Q1 2015 period end rates for the rest of 2015, it is estimated that there would be no
material currency impact on 2015 sterling turnover or core EPS
* The other currencies that each represent more than 1% of
Group sales are: Australian Dollar, Brazilian Real,
Canadian Dollar, Chinese Yuan, Indian Rupee. In total
they accounted for 13% of Group revenues in 2014.
2015 core EPS ready reckoner 2014 currency sales exposure*
* 2014 legacy GSK.
77
