Smart Pharma Consulting
Strategic KOL
Engagement
Planning…
Smart Pharma
Consulting
… For a better
Efficacy & Efficiency
Position Paper
May 2019
Concepts
Methods
&
Tools

Smart Pharma Consulting
Table of Contents
May 2019
2
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
1. Introduction p.3
2. Strategic KOL Engagement Planning p.4
Introduction p.4
Benchmarking study p.6
A 4-step approach (Introduction) p.14
̶ Objective setting p.16
̶ KOL selection p.19
̶ KOL engagement p.27
̶ KOL engagement & monitoring p.37
3. Conclusions p.41
Smart Pharma Consulting
Sources: Smart Pharma Consulting
1. Introduction
1
In this position paper, the definition of KOL is limited to influential physicians
This position paper proposes guidelines to help pharmaceutical companies partner
with KOLs to better support the development and the marketing of their products
Context & Objective
KOLs
1
are part of the means used by pharma companies to:
– Develop their products through pre-clinical and clinical trials
– Disseminate information (scientific, medical, therapeutic, etc.) to raise health authorities,
payers, HCPs (Health Care Professionals), PAGs (Patient Advocacy Groups), individual
patients awareness to optimize the positioning and the usage of their products
This position paper:
– Reviews the best practices in terms of KOL engagement
– Proposes a simple but rigorous approach and…
– … a set of practical tools…
… to recruit, engage and manage KOLs
This position paper has been written, assuming that it is not illegal nor reprehensible to collaborate
with medical thought leaders to influence other stakeholders opinion and behavior vis-à-vis
a medical practice or a given medicine, provided it is in the best interest of patients
May 2019
3
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Smart Pharma Consulting
Pyramid of influence & types of influencers
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning Introduction
May 2019
4
KOLs have the potential to influence their peers, but also other stakeholders in a
specific area, at global, international, national and local levels
Working definitions (1/2)
KOLs are also called: Key Experts, Key Therapeutic
Area Experts, Key Scientific Experts, Thought
Leaders, Influencers, depending on the companies
KOLs are recognized physicians with an expertise
in a specific field (e.g. oncology, endocrinology,
epidemiology, biostatistics, etc.)…
… and can influence the opinion and the medical
practice (e.g. treatment scheme, prescribing habits,
preference for a given product, etc.) of their peers
(specialists or GPs)
KOLs contribute also to modify medical guidelines
when they are members of learned societies or when
they advise health authorities
Their influence can be global, international, national
or local
Other stakeholders are also considered as KOLs
1
KOL (Key Opinion Leader)
International
KOLs
National & Local KOLs
Practitioners (specialists or GPs)
Learned
societies
PAGs
Nurses Pharmacists
Journalists
Politicians Government
Health
authorities
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Other stakeholders than physicians likely to have an influence
on medical practices and on physicians opinion and behavior
1
Such as members of governments, of health authorities, of learned societies,
of patient advocacy groups, journalists, pharmacists, nurses, etc.
Global
KOLs

Smart Pharma Consulting
Sources: Roche internal documents (2015) – Smart Pharma Consulting
2. Strategic KOL Engagement Planning Introduction
May 2019
5
Strategic KOL Engagement Planning is essential for pharma companies to ensure an
effective, efficient and sustainable relationship with KOLs
Working definitions (2/2)
KOL engagement is a process in which pharma
companies build and maintain constructive and
sustainable relationships with KOLs
KOL engagement is essential for understanding
their wants and needs; and may result in
implementing ideas that benefit both KOLs and
pharma companies
Engaging with KOLs occurs when pharma
companies want to consider the views and
involvement of KOLs in making and implementing a
scientific or medical decision…
… which might have an indirect business impact
Pharma companies should initiate open, two-way
dialogue, seeking solutions to issues of mutual
interest
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
1
People from different departments (e.g. medical, marketing, sales, etc.)
can be in regular contact with the same KOL
Considering the increasing complexity of the
pharmaceutical environment and of pharma
companies organizations
1
, it is essential to plan and
organize the interactions with KOLs
Thus, pharma companies should develop Strategic
KOL Engagement Plans to ensure, as a general
rule, that KOL Engagement initiatives:
‒ Support the Critical Success Factors (CSF) to
fulfill the corresponding Strategic Imperatives
(SI) of the related product
‒ Are put in a mid- to long-term perspective to
build a sustainable win-win relationship
‒ Are carried out in a coordinated manner across
the company departments and from headquarter
to affiliates to guarantee an optimal efficiency
Strategic KOL Engagement Planning KOL Engagement
Smart Pharma Consulting
Sources: Arx Research (2017) – Smart Pharma Consulting analyses
2. Strategic KOL Engagement Planning Benchmarking study
More and more pharma companies are adopting an integrated strategic approach of
their relationship with KOLs, based on their product position on their life cycle
Types of KOL engagement
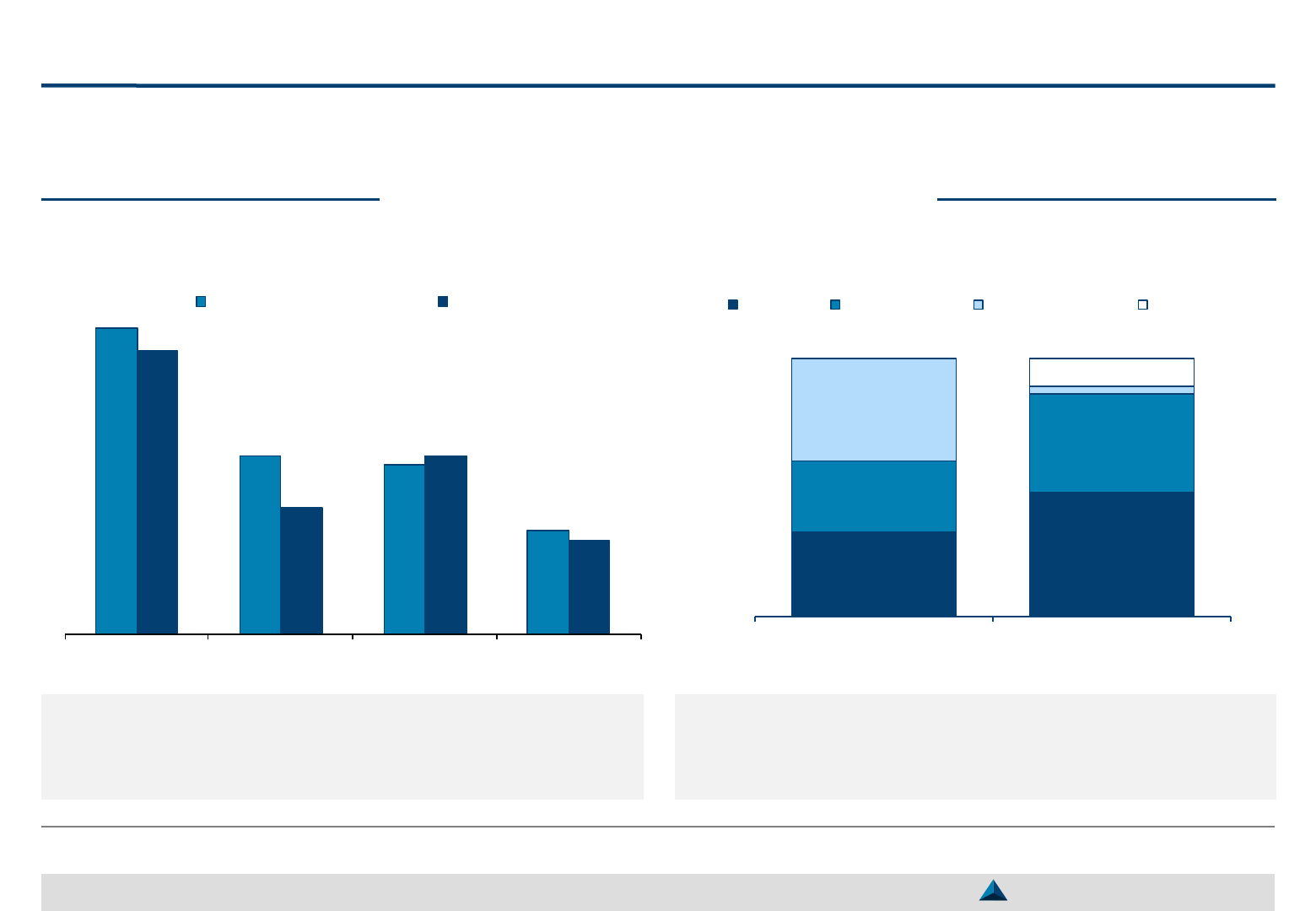
According to a study carried out in 2017 by Arx
Research, through interviews of 47 executives from
medical departments of 34 life science organizations,
across 15 countries:
– 70% of companies indicate that their strategy to
engage with KOLs is based on the position of the
product on its life cycle, while the remaining 30%
adopt an ad-hoc approach
– 24% of surveyed companies engage with KOLs
during pre-clinical phases of the product
development and…
– … 41% begin developing relationships at phase III
of their product life cycle, or after
KOLs exposed to early research and development
phases will better support the products due to:
– A better understanding of the underlying science
– A better commitment and interest in outcomes
May 2019
6
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
70%
30%
Ad-hoc
approach
Product
lifecycle- based
approach
KOL engagement approach
KOL engagement according to product lifecycle
41%
35%
24%
Clinical phase III & launch
Clinical phases I & II
Pre-clinical phases

Smart Pharma Consulting
Sensitivity level
Sources: Arx Research (2017) – Smart Pharma Consulting analyses
2. Strategic KOL Engagement Planning Benchmarking study
The strength of KOL engagement will strongly depend on the quality of scientific
evidence related to the product as well as on corporate and product perception
KOLs engagement & Influencing factors
May 2019
7
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Global & International KOLs
engaged at Phase III and Launch phase
Factors influencing KOL relationships
8,00
5,86
5,74
4,95
4,91
0 2 4 6 8 10
Quality of scientific
evidence
Corporate reputation
Product recognition
Therapeutic area
Competitive landscape
56%
44%
Phase III
Launch phase
Low High
From preclinical to phase II studies, Global KOLs are
engaged to carry out scientific and clinical activities
At phase III level, Global, International and National
KOLs are mainly involved in clinical studies and in
disseminating scientific information to physicians
communities
While preparing the launch of their products or of new
indications, pharma companies may engage KOL to
support the preparation of the marketing authorization
and of the price & reimbursement dossiers
At launch time, pharma companies usually shift the
balance of their focus to national and local KOLs
The quality of the scientific evidence is critical to
establish strong and effective relationships with KOLs
Corporate reputation and product recognition are also
essential to expect a clear commitment from KOLs
Smart Pharma Consulting
Sources: Best Practices, LLC (2014 & 2016) based on 33 companies, amongst which: AbbVie, Amgen,
Bayer, Genentech, Genzyme, Janssen, Merck & Co, Pfizer, Roche – Smart Pharma Consulting analyses
2. Strategic KOL Engagement Planning Benchmarking study
* One respondent considers there is no ideal system
to manage KOLs. It depends on the business needs
The hybrid and centralized management of KOLs are viewed as optimal by
interviewees as they enable better coordinated and more consistent interactions
KOLs management by pharma companies
Functional and budget responsibility for KOL
management are mainly in the hands of Medical
Affairs departments
May 2019
8
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
KOL Management organization at pharma companies KOL Management responsibility at pharma companies
Decentralized organizations are used by 40% of
companies but recommended by only 3% of them
due to lack of coordination and consistency
94%
55%
52%
32%
87%
39%
55%
29%
Medical Affairs Clinical
Affairs/Operations
Marketing R&D
Functional Budget
N=30
33%
48%
27%
38%
40%
3%
11%
Current Optimal
Hybrid Centralized Decentralized Other*
N=33 N=29
Smart Pharma Consulting
Sources: Best Practices, LLC (2014 & 2016) based on 33 companies, amongst which: AbbVie, Amgen,
Bayer, Genentech, Genzyme, Janssen, Merck & Co, Pfizer, Roche – Smart Pharma Consulting analyses
2. Strategic KOL Engagement Planning Benchmarking study
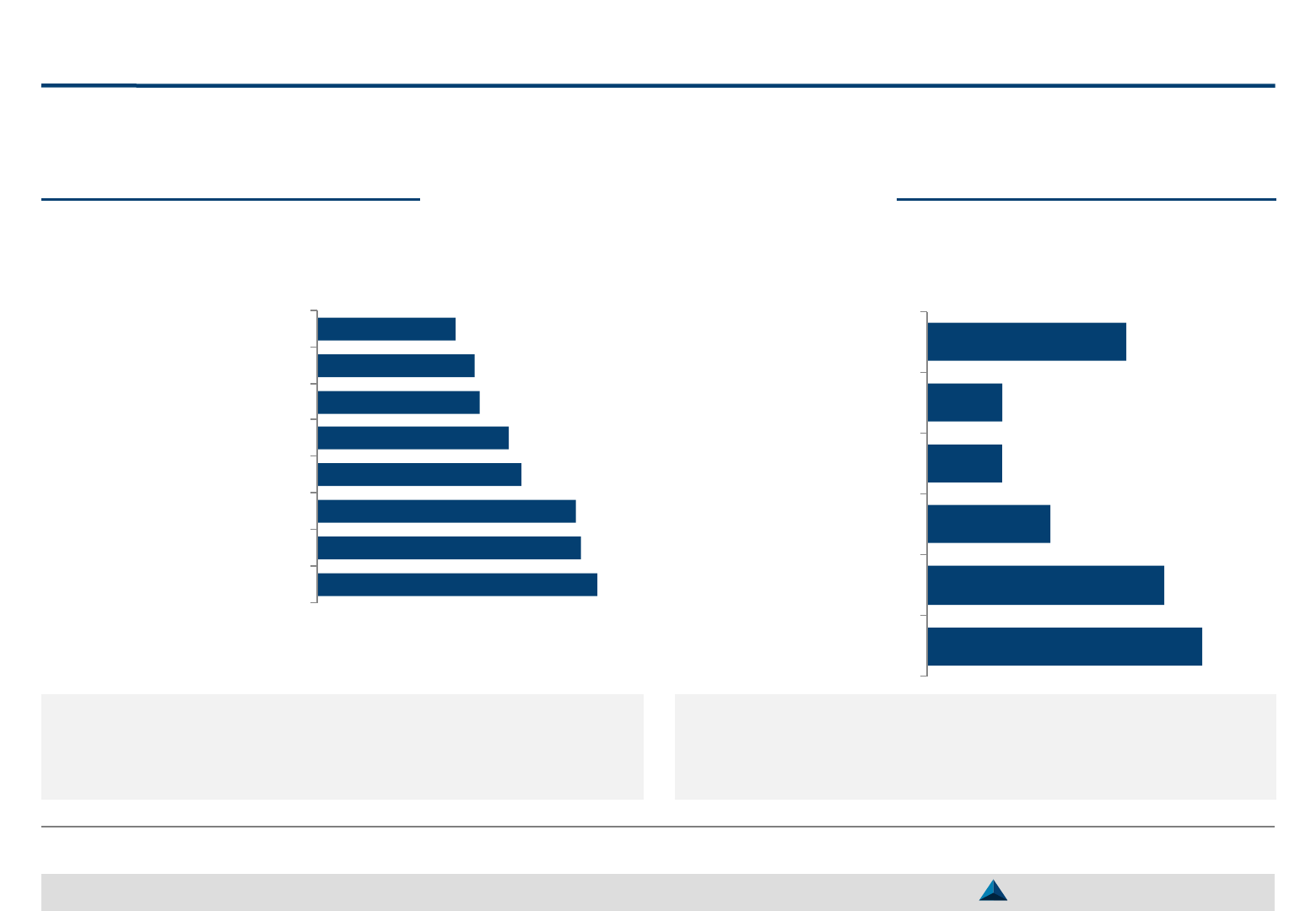
If KOLs services are mainly focused on clinical research, clinical advisory boards and
disease state awareness exchanges; their impact is most often not formally evaluated
Main KOLs services & assessment
Clinical research support, participation to medical
advisory boards and disease state awareness are
viewed as the most important KOLs activities
May 2019
9
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Evaluation of KOL Management & Engagement Most important services carried out by KOLs
There is no formal nor systematic measurement of
the impact of KOLs engagement carried out by
most of the pharma companies from the panel
N=28 N=24
114
107
105
83
78
66
64
56
Clinical research
Medical advisory boards
Disease state awareness
Peer-to-peer presentations
Speaker training
Off-label discussion
Consulting opportunities
Marketing advisory board
29%
25%
13%
8%
8%
21%
No formal evaluation
KOL feedback
No evaluation
KOL utilization level
Return on investment
Other
Note: Score based on the average importance rating (0 to 5) multiplied by the
number of respondents per activity
Mean score: 75
Smart Pharma Consulting
Sources: Interviews of 8 Senior Medical executives from Bayer, BMS, Celgene, Gilead, Janssen, MSD, Pfizer, Roche
– Smart Pharma Consulting analyses
2. Strategic KOL Engagement Planning Benchmarking study
1
Therapeutic Area
Few of the 8 benchmarked pharma companies have put in place a systematic and
formalized process to qualify and select Global KOLs
Global KOLs qualification & selection
May 2019
10
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
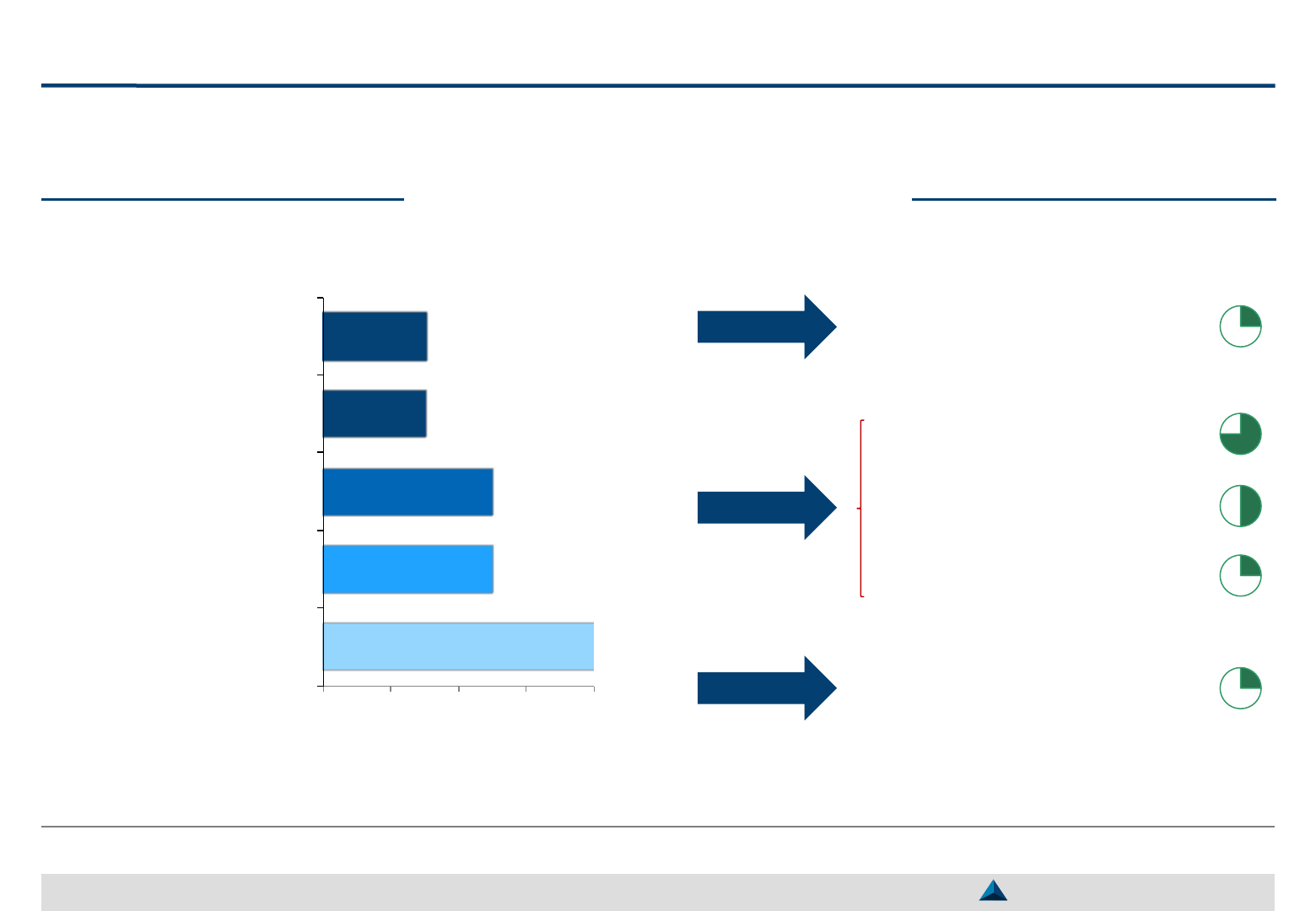
Main criteria to select Global KOLs
8
5
5
3
3
0 2 4 6 8
Publications
Involvement in the crafting
of guidelines
Scope of influence /
Reputation
Membership to cooperative
groups
Skills
Note: Behavior & personality has been mentioned by
one interviewee, as well as KOLs field of interest
Data gathering
Process
Formal process
Sources
External agency if new TA
1
Tools
Centralized database
Inputs from affiliates
Internal / external cross-check
“In case of doubts, Global Medical Affairs may contact local
Medical Affairs to get their own opinion regarding a Global KOL”

Smart Pharma Consulting
Sources: Interviews of 8 Senior Medical executives from Bayer, BMS, Celgene, Gilead, Janssen, MSD, Pfizer, Roche
– Smart Pharma Consulting analyses
3. Strategic KOL Engagement Planning Benchmarking study
1
Several answers possible
According to the spontaneous statements of interviewees, Global KOLs are mainly
engaged to give advice on brand positioning, produce and exchange scientific data
Main objectives while engaging with Global KOLs
May 2019
11
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Main objectives
1
5
3
-2 3 8
Brand positioning
Data generation &
exchange
Objective setting
No formal approach, based on specific KOL expertise
and company needs
“While engaging with a KOL, we make sure he is interested
by the project on which we want to involve him”
Objective alignment on product Strategic
Imperatives & Critical Success Factors
No formal alignment / no global vision
Alignment on Global Strategic Brand Plan / R&D Plan /
Global Medical Affairs Plan
Type of agreements
3
2
3
0 1 2 3 4 5
Mainly transactional
Mainly Partnership-based
Both types
Smart Pharma Consulting
Sources: Interviews of 8 Senior Medical executives from Bayer, BMS, Celgene, Gilead, Janssen, MSD, Pfizer, Roche
– Smart Pharma Consulting analyses
2. Strategic KOL Engagement Planning Benchmarking study
Global KOL engagement plans are most often not formalized for each KOL and their
follow-up over time is far from being systematic
Global KOL engagement planning & execution follow-up
May 2019
12
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Global KOL engagement plans Execution quality follow-up
“We prepare an engagement plan but by project rather
than by KOL. We engage a KOL to carry out a project”
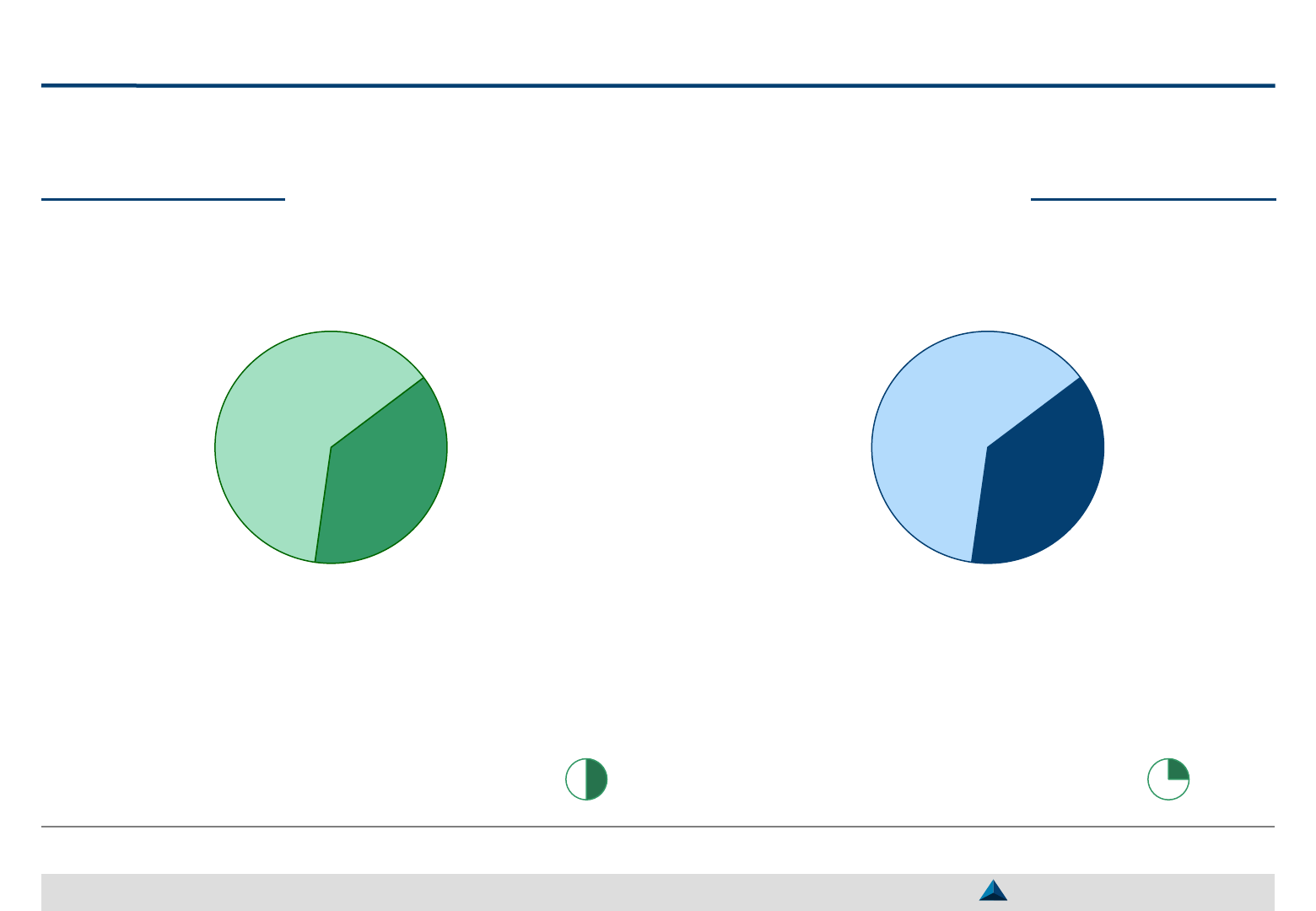
Yes
3
No
5
Yes
3
No
5
“In Europe, it is difficult to evaluate the performance of KOLs.
It should be fact-based and not a judgement”
System to monitor the implementation
of Global KOL engagements
Main difficulties while engaging with Global KOLs
Poor internal alignment and multiple contact points Overbooked and overused KOLs
Smart Pharma Consulting
Sources: Best Practices, LLC (2014 & 2016) based on 33 companies, amongst which: AbbVie, Amgen,
Bayer, Genentech, Genzyme, Janssen, Merck & Co, Pfizer, Roche – Smart Pharma Consulting analyses
2. Strategic KOL Engagement Planning Benchmarking study
1
Whenever required by the compliance rules –
2
Internal and external sources
The effective KOL management requires a cross-functional team working in the same
direction, in a coordinated manner, with the help of a shared information system
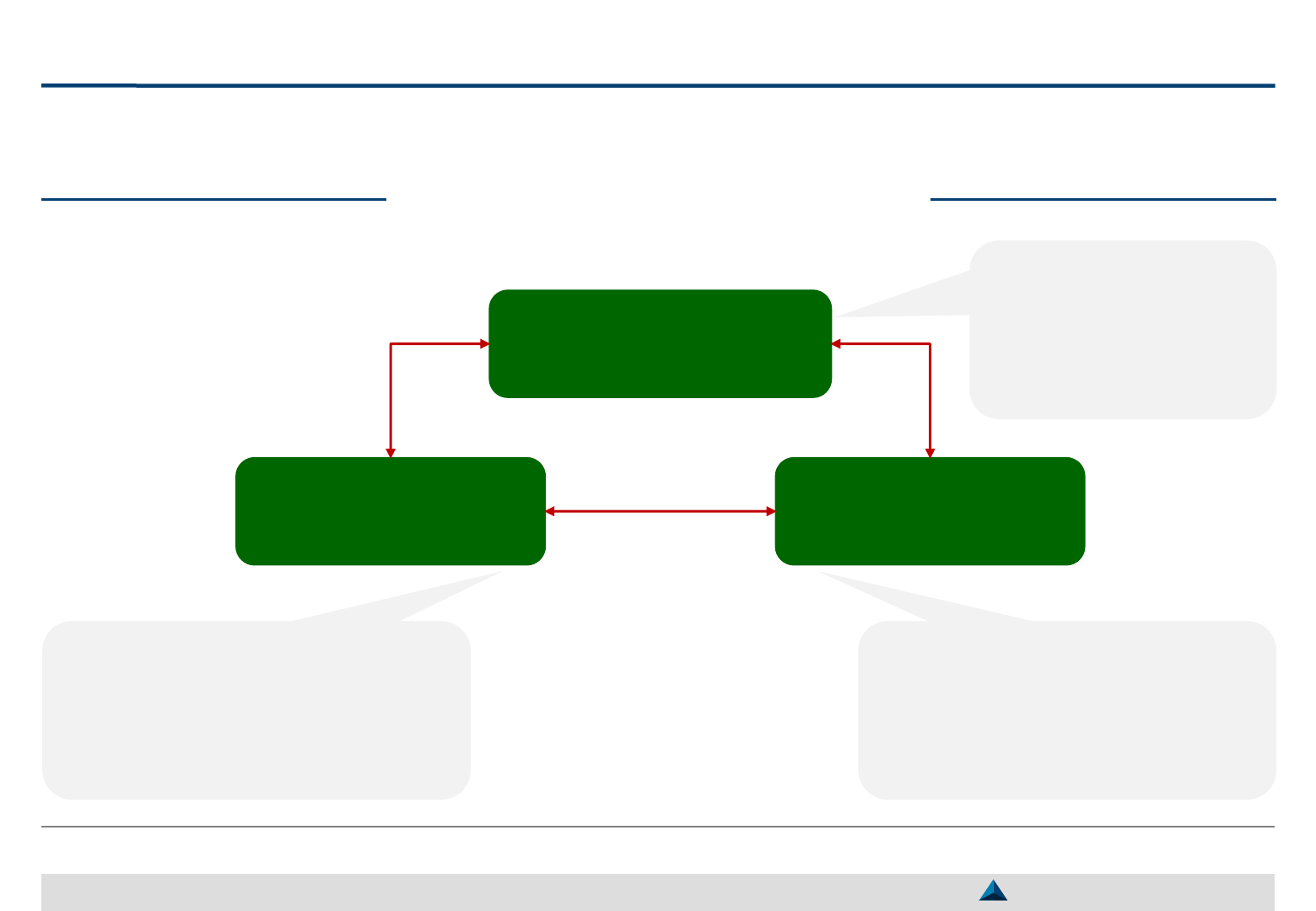
Strategic KOL Management components
May 2019
13
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Coordinator
(Primary Contact Point)
KOL Management
System & Platform
Cross-functional
Strategic Team
Coordinates interactions
with KOLs
Oversees the management
system
Guarantees the quality of
the collaboration
Gathers and analyzes information
2
across access, medical and commercial
departments
Prioritizes the activities to be carried out
by individual KOL according to the
product needs and the KOL profile
Stores information relative to:
̶ KOLs profiles
̶ KOL engagement plans
̶ KOL interactions
Limits the access of certain medical
information to commercial collaborators
1
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning A 4-step approach (Introduction)
May 2019
14
The following 4-step approach is proposed to ensure an effective and efficient
Strategic KOL Engagement Planning
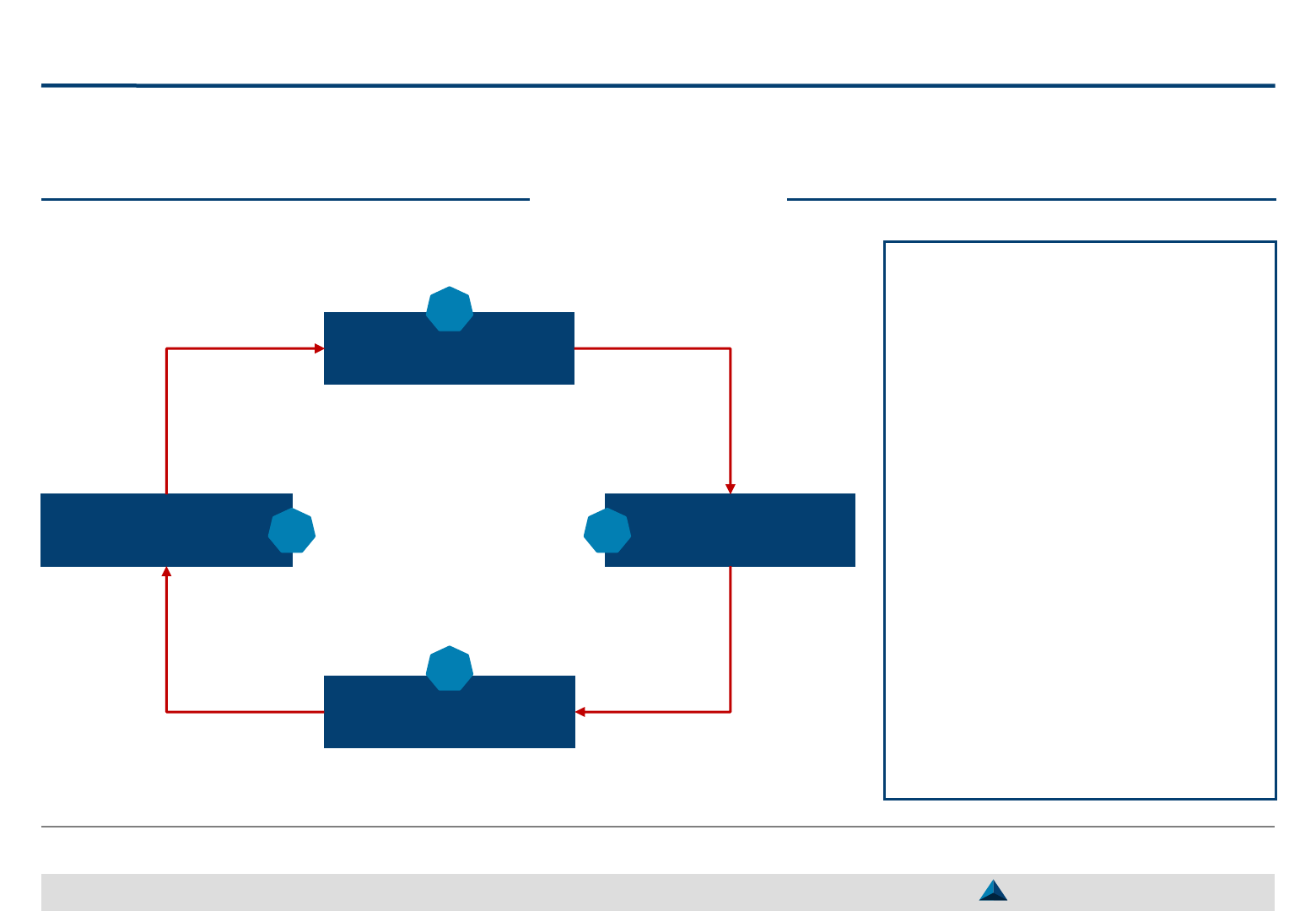
A 4-step approach
Objective setting
1
KOL Selection
2
KOL Engagement
3
Monitoring
4
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Relationships with KOLs should
be defined according to the set
objectives
Then, the prospective KOLs
should be profiled and targeted
Once KOLs have been selected,
their interactions with the pharma
company and the activities they
are expected to carry out should
be defined and formalized in an
engagement plan
The execution of the plan should
be carefully monitored with the
help of KPIs (Key Performance
Indicators) and of KEIs (Key
Execution Indicators)
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning A 4-step approach (Introduction)
May 2019
15
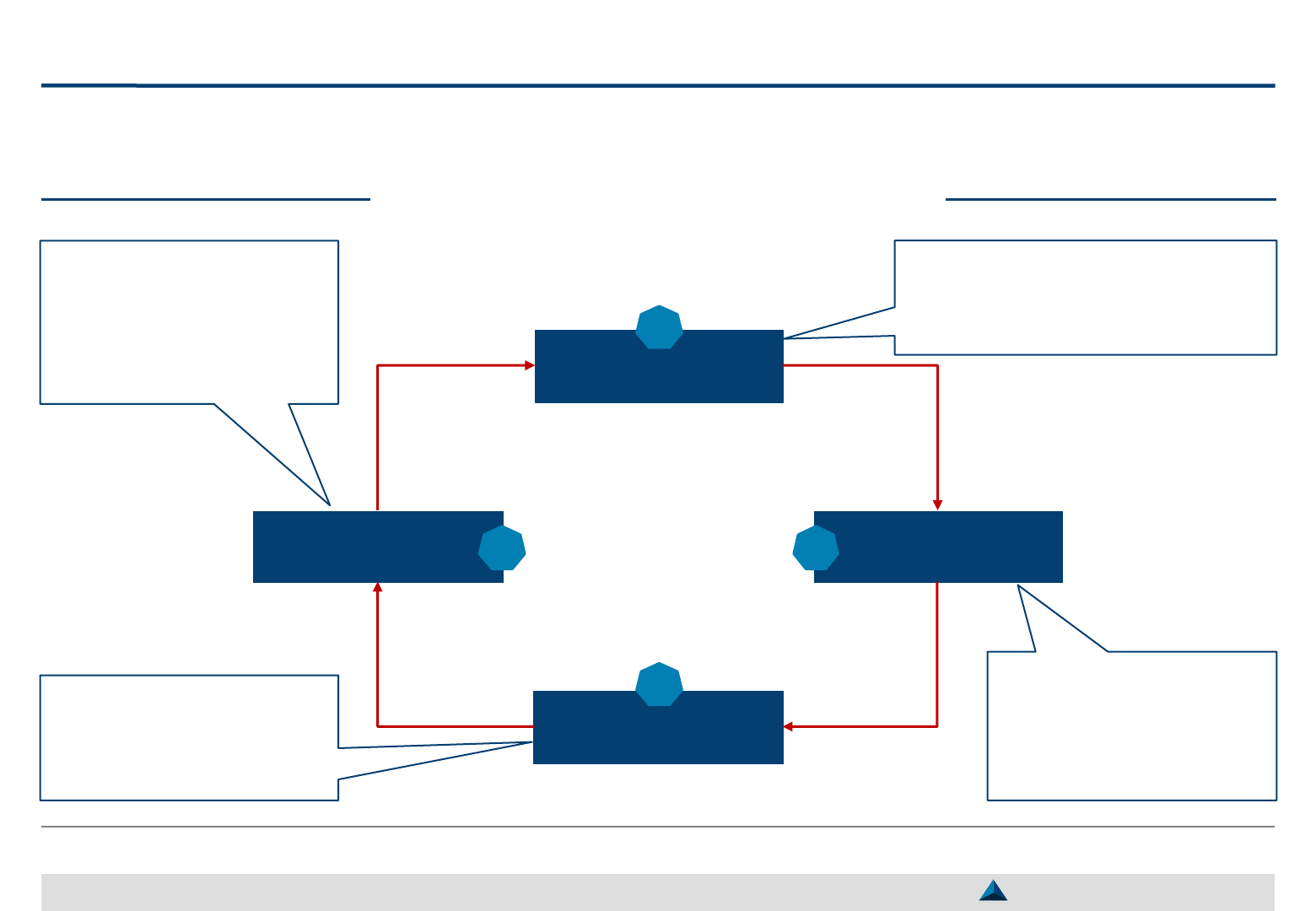
At each step, the following key questions should be carefully answered to ensure the
proper implementation of the proposed Strategic KOL Engagement Planning process
Key questions to be answered by key step
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
What do we expect from KOL
engagement?
What do KOLs expect from us?
Who are the KOLs we
want to engage with?
Why do we want to
engage with them?
Which interactions
should be carried out
with KOLs to reach the
set objectives?
How to measure the
impact of KOL
engagement vs. the
objectives and KOL
satisfaction?
Objective setting
1
KOL Selection
2
KOL Engagement
3
Monitoring
4
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning Objective setting
16
1
Critical Success Factor
The global objectives set for KOL engagements should contribute – directly or
indirectly – to meet the brand strategic objectives, irrespective of its life cycle position
Strategic alignment
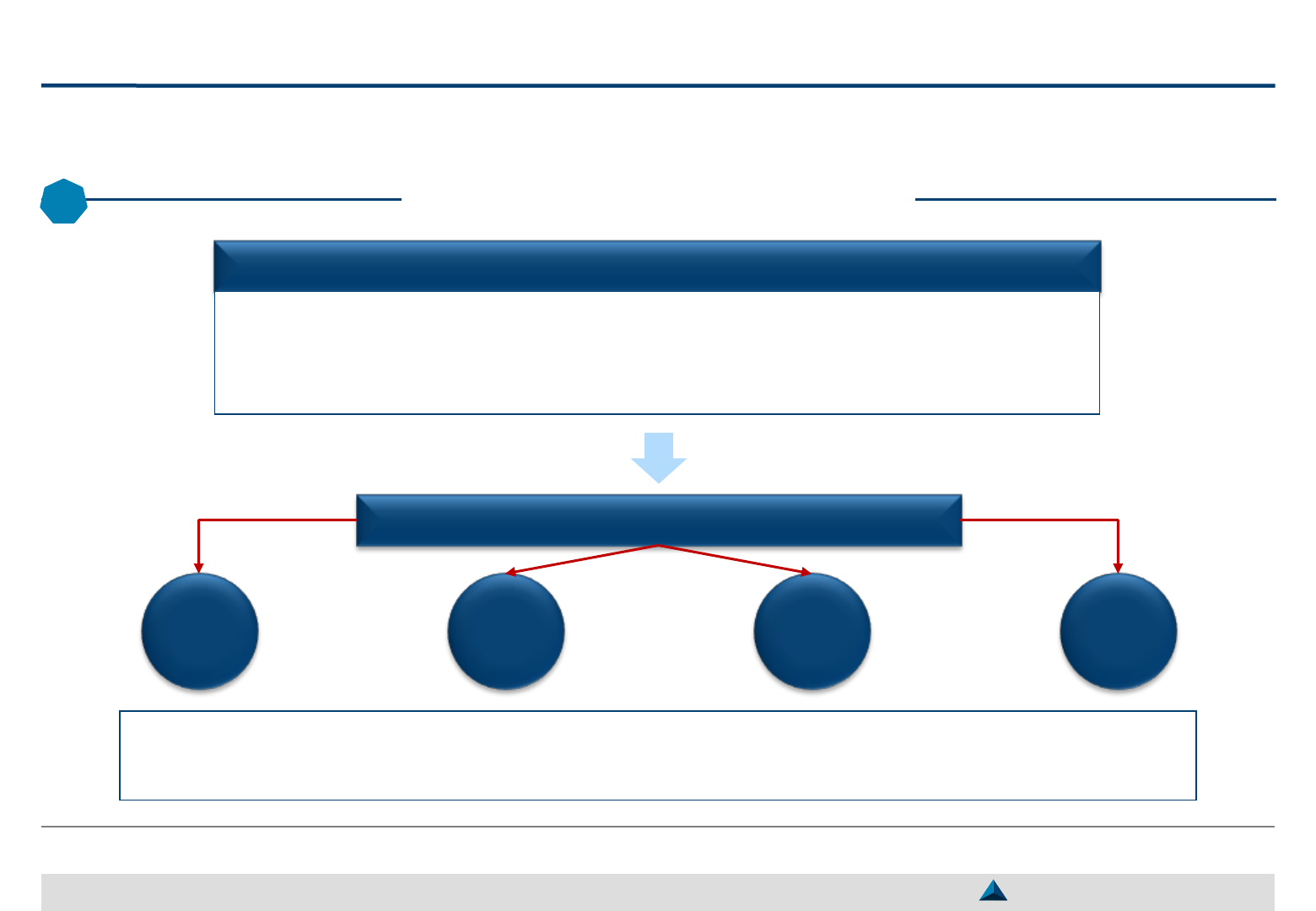
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Brand Strategic Objective
Strategic Imperative
A
Strategic Imperative
B
Strategic Imperative
C
CSF
1
A1
CSF A2
CSF A3
CSF B1 CSF C1
CSF C2
CSF C3
CSF B2
The global objective of KOL engagements must support
one or several CSFs and thus, contribute to fulfill
the strategic imperatives to reach the Brand Strategic Objective
Strategic Brand Plan
(2020 – 2023)
1
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning Objective setting
17
Before defining the KOL Engagement Plan, specific objectives by KOL, consistent
with the Brand Strategic Objective, must be set
Global vs. individual objective setting
KOL 1
1
May 2019
Global objectives
KOL 2 KOL 3 KOL 4
Define precisely what is expected from KOL engagement,
in terms of direct or indirect benefits for the brand
under development or marketed by the pharma company
Individual objectives set by KOL
Define specifically what is expected from each KOL to support the product
and what support each KOL expects from the pharma company, on a professional stand point
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Smart Pharma Consulting
Sources: Adapted from GBI Research, Market Rx, by Smart Pharma Consulting
2. Strategic KOL Engagement Planning Objective setting
1
Through articles, lectures, etc. –
2
Through Continuous Medical Education (CME) programs –
3
Through projects carried out with patient advocacy groups (PAGs) –
4
Investigator Initiated Trials
Examples of objectives along the product life cycle
1
The objective of the KOL partnership and the corresponding activities will depend on
where the product is positioned on its life cycle
Research & pre-
clinical phases
Phase
II
Approval
Phase
I
Phase
III
Pricing &
reimbursement
Marketing
Product life cycle
Examples of KOL roles
Identification of
pharmacological
targets
Identification of unmet
medical needs
Presentation of clinical results
and of product benefits
to regulators and payers
18
May 2019
R&D and registration phases Commercial phase
Implementation of R&D activities
Product awareness
building & influence on
prescribing choices
1
Participation in medical
education programs
2
Contribution to patient
management programs
3
Advice on target product
profile and labelling
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Real World Data generation/
phase IV studies
IIT
4

Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL selection
1
Collects and collates disparate information on the online activity surrounding scholarly content
The selection phase consists in a 4-step process leading to a pool of KOLs with
whom to engage to benefit (directly or indirectly) the brand
Methodology
2
Selection criteria
KOLs segmentation
KOLs selection
KOLs profiling
What are the relevant selection criteria to
be used considering the final objective?
What information should be collected?
How to collect and analyze this
information?
What is the scope of influence and the
degree of interest of the KOL for the
brand and the related disease(s)?
Key questions What to do?
Review the relevant criteria (e.g. level of influence,
scope of influence, scientific/media awareness,
membership of a network, presence in Internet, etc.)
Select a limited number of relevant criteria
Review internal / external databases to qualify KOLs
Assess the number of publications, quality of journal,
the impact factor, Almetrics
1
, quotes, lectures during
conferences and congresses, etc.
Map a preselection of KOLs on a matrix according to
the most relevant criteria
Identify KOLs networks of collaboration and
influence (e.g. cooperative groups)
Who are the KOLs that should be
engaged?
For which kind of engagement?
Select the KOLs
Preliminarily define the types of engagement to carry
out with the selected KOLs
19
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL selection
1
Patient advocacy groups
Relevant selection criteria and gathering of accurate and reliable information about
the KOLs profiles are of utmost importance to optimize the value of their engagement
Screening process (illustrative)
2
Discarded
physicians
Pre-selected
physicians
# of
specialists
Filter 1
Field of expertise
Filter 2
Level of reputation
& influence
Inclusion criteria
Oncology (medical, radiation and surgical
oncology, hematology, brain cancer, etc.)
Cardiology (hypertension, arrhythmias, heart
failure, surgery, valvopathy, etc.)
Rheumatology (osteoporosis, rheumatoid
arthritis, osteoarthritis, psoriatic arthritis, etc.)
Technical expertise (design of clinical
studies, biostatistics, epidemiology, public
healthcare, patients adherence, etc.)
Inclusion criteria
Reputation of the hospital/ward
the KOL works for
Reputation of the KOL (based on
status, honors, publications, etc.)
Power of influence (on peers,
health authorities, PAGs
1
)
Scope of influence (global,
international, national, local)
Discarded
physicians
Pre-selected
physicians
Filter 3
Potential interest
Pre-selected
physicians
Inclusion criteria
Inclination to communicate
(in a neutral or positive way)
Communication skills
(written and/or verbal)
Discarded
physicians
20
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL selection
21
1
Medical Science Liaisons
Qualification of KOLs should be documented with reliable and real-time data collected
through desk research and field research (e.g. interviews of peers, pre-identified KOLs)
How to qualify KOLs? (1/2)
2
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
What data to collect? How to collect data? How to analyze data?
Education
(e.g. university – hospital)
Medical activity/position
(e.g.
specialty, medical department, status
in the medical department)
Teaching activity/position
(e.g. topics taught, professor, lecturer)
Field of expertise and interest
(e.g. specific disease, pharmacological
route, mode of action, medical technique)
Membership in learned
societies
(titles / positions / activities) and/or in
more or less structured networks
Internet search, direct search
Field research (e.g. peers,
hospital pharmacists
interviews, etc.)
Probing by collaborators from
the medical department (e.g.
MSLs
1
) and
collaborators from
other departments of the
pharma companies (data could
be stored and shared on a
platform)
KOL Management vendors
(e.g. Truven; KOL, LLC;
OpenQ; Veeva Systems)
Being head of hospital and professor
is
a plus
Reputation of the hospital/teaching
hospital or of the private institution
where the KOL works should be
considered
Global or International scopes of
influence are preferable, in general, to
national or local levels (but it depends
on the objective)
Being a member of the management
board of a learned society is a plus in
terms of potential level of influence
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL selection
22
1
It measures the average frequency with which the article has been cited in a particular year. It is used to measure the
importance or rank of a journal by calculating the number of times its articles are quoted –
2
Collects and collates
disparate information on the online activity surrounding scholarly content –
3
Continuous medical education
Qualification of KOLs should be documented with reliable and real-time data collected
through desk research and field research (e.g. interviews of peers, pre-identified KOLs)
How to qualify KOLs? (2/2)
2
May 2019
What data to collect? How to collect data? How to analyze data?
Communication activities
– # articles published (impact factor
1
,
Almetrics
2
, peer-/non peer reviewed
journals, principal investigator (PI), etc.)
– # of training/teaching activities p.a.
(CME
3
)
–
# of lectures (congresses, symposiums,
round tables)
– Presence on the Internet
–
# of quotes by journalists in current year
Review of published scientific
articles (PubMed/Medline, Google
scholar, Expertscape, Cochrane Library)
Evaluation of training/teaching
activities and lectures by
interviewing peers and
collaborators of pharma
companies
Google searching for presence
and quotes on the Internet
The higher the impact factor is,
the
better
KOLs should be ideally positioned
as 1
st
or last author in articles
A high number of training/teaching
seminars and lectures is a plus
The perceived quality of articles,
training, teaching and lectures
should be assessed
Partnership activities
–
Types of activities (e.g. lectures, clinical
investigations, advisory boards)
– With the company and its competitors
– Potential level of interest (inclination to
support the development/the proper use
of a brand)
Review of past performances with
the company or its competitors
(e.g. probing by collaborators of
the company)
Interviews of peers
Verbal (e.g. lectures, courses) and
written communication (e.g. articles,
websites)
KOLs should express their field of
interest over the long term and their
expectations from an engagement
with the pharma company
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Smart Pharma Consulting
Sources: Niche Science & Technology (2016) –
Smart Pharma Consulting analyses
2. Strategic KOL Engagement Planning KOL selection
23
1
Average of the marks obtained –
2
[Expertise x Awareness] / 2
The following table shows a proposed approach to evaluate and rank candidate
KOLs to set up a list of Top Global KOLs, that should be continuously updated
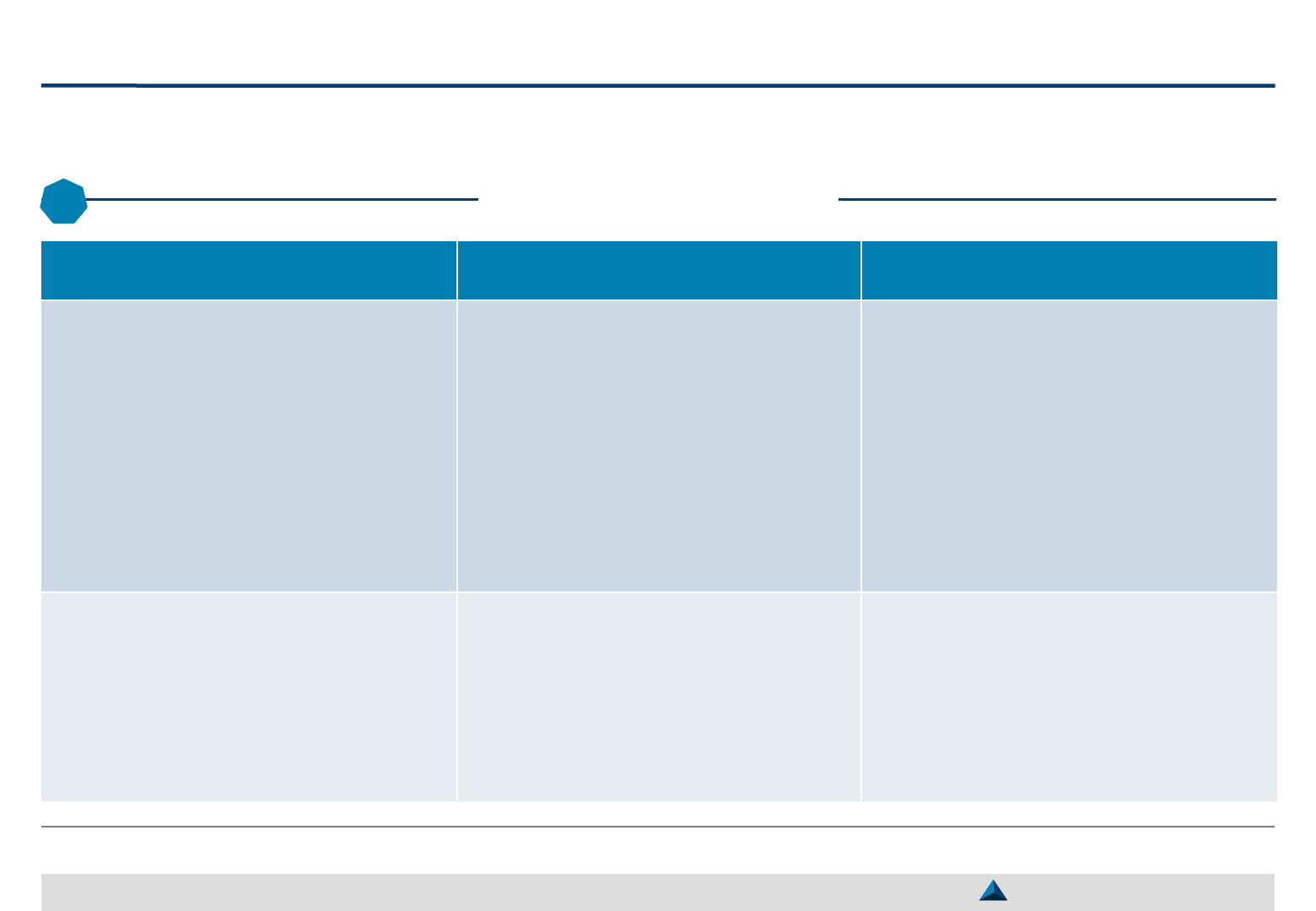
Scoring of candidate KOLs
2
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Illustrative
Profiling parameters Prof. A
Prof. B
Prof. C
Dr. D
EXPERTISE
Pharmacological expertise
8 0 6 0
Academic research
5 9 0 0
Clinical research
5 0 9 5
Clinical practice
0 0 6 9
Scientific advisory board
8 8 7 6
Sub-total score (A)
1
5.2 3.4 5.6 4.0
AWARENESS
Publication record
8 5 4 3
Speaker record
3 4 8 7
Communicate skills
6 6 5 7
Density of the network
5 7 7 3
Sub-total score (B)
1
5.5 5.5 6.0 5.0
Impact Index
2
score (A x B)
1
14.3 9.4 16.8 10.0
KOL degree of interest
Moderate
High
Moderate
Low
Ranking
2 3 1 4
The candidate KOLs can be ranked
according to their field of expertise,
their associated level of recognition
in these fields, and their level of
awareness
The KOL degree of interest for the
product should also be considered
The assessment could be done on
a 10-point scale based on data
coming from external providers, a
panel of peers who will score each
expert, combined with the internal
insights available at the pharma
companies level, etc.
This approach will help make a first
cut of the Top Global KOLs that
should be continuously reevaluated
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL selection
1
Including on Internet –
2
Network of influence / collaboration amongst KOLs
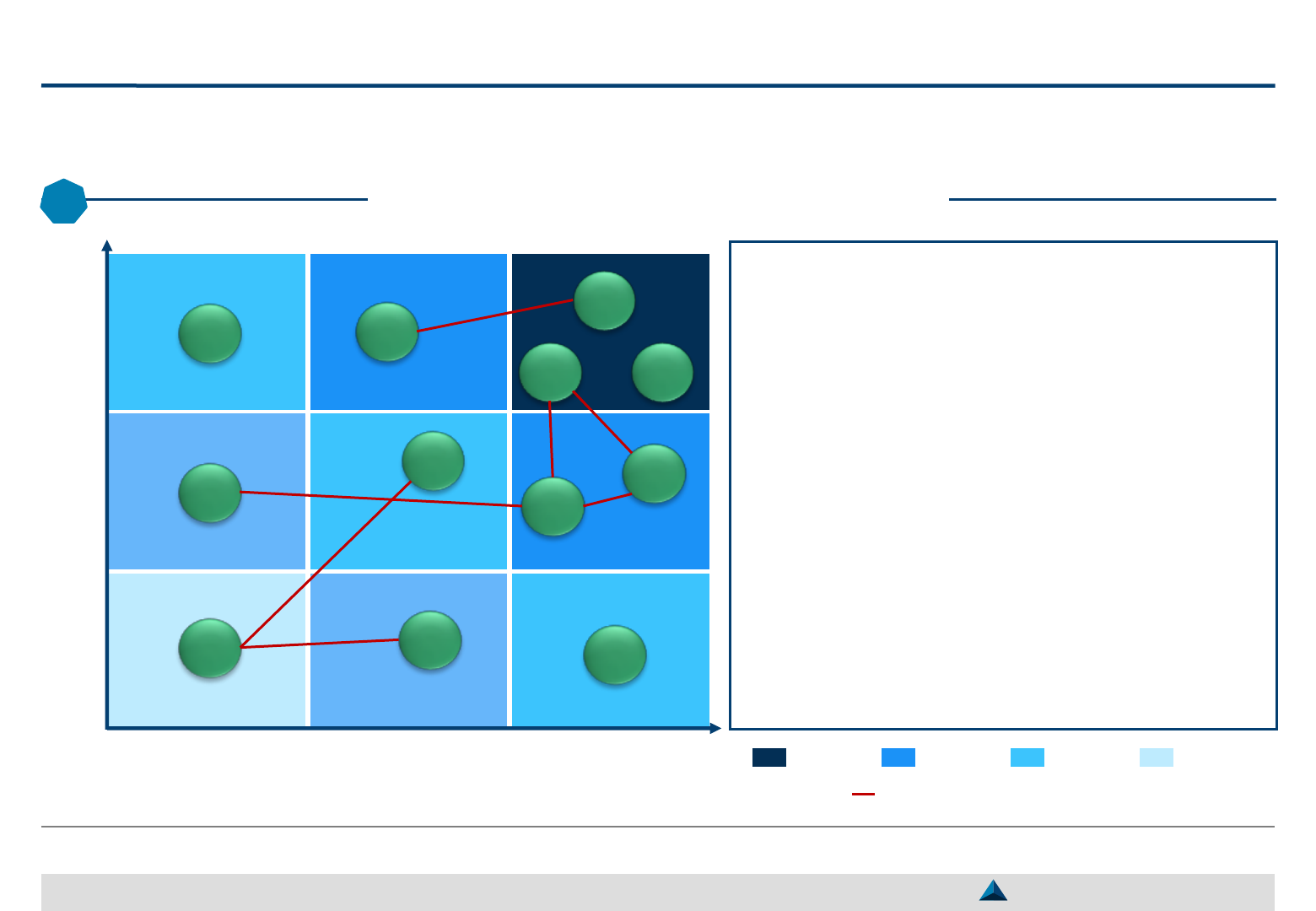
The proposed matrix is a useful tool to prioritize the KOLs with whom to engage and
to pre-define the types of collaboration to carry out with them
KOL targeting – Segmentation & selection
2
Low Moderate High
Impact Index (Expertise x Awareness)
Low Moderate High
Degree of Interest
K
L
E
A
D
C
H
I
F
B
24
May 2019
G
J
Priority 1 Priority 3 Priority 2 Not a Priority
The proposed matrix facilitates the final
selection (targeting) of pre-selected KOLs
based on their:
̶ Impact index (combining their degree of
expertise and awareness
1
)
̶ Potential interest
The impact index reflects the KOLs ability to
influence other stakeholders (i.e. HCPs, policy
makers, payers, patients, PAGs)
The degree of interest reflects the KOLs
willingness to support:
– The development of the company brand
– The proper use of the brand, once marketed
The network
2
of KOLs should also be considered
Networks of influence / collaborations amongst KOLs
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL selection
1
Investigator Initiated Trials–
2
Especially for Rising Opinion Leaders
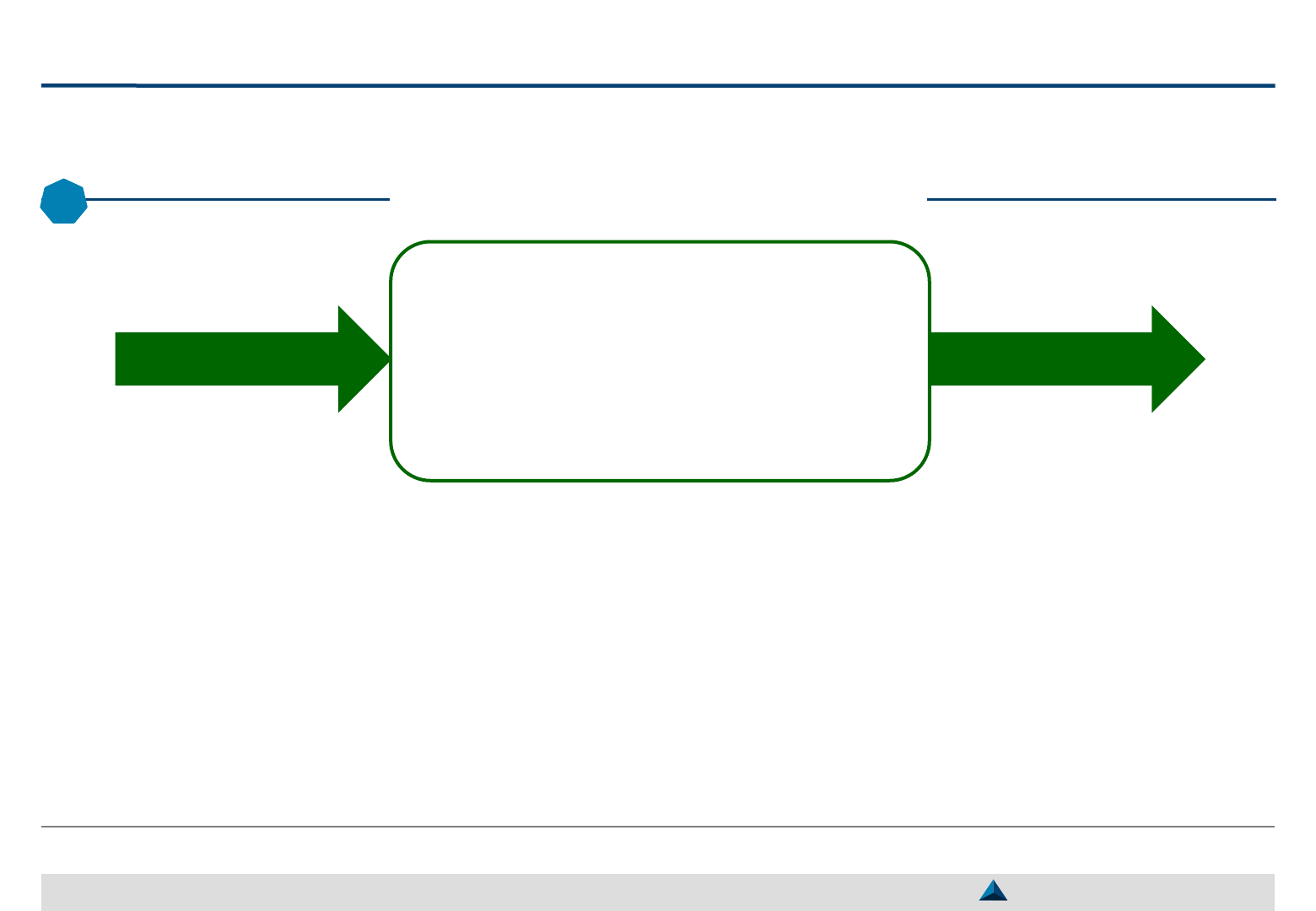
To convince KOLs to partner, it is important to consider their expectations and to
highlight the benefits they will draw from it in terms of professional development
How to convince KOLs to partner?
May 2019
The selection of KOLs should consider the benefits
they can offer to the pharma companies and the
benefits the pharma companies can offer to them
For so doing, the following questions should be
addressed:
– Is the KOL yet a partner of the pharma company?
– What has been qualitatively and quantitatively his level of
involvement?
– What has been his feed-back (level of satisfaction) from
previous collaborations?
– What is his mid- to long-term professional ambition?
– What does he expect from collaborations with pharma
companies?
– Is he looking for a long-term partnership or a “fee-for-
service” transaction?
Based on KOLs professional expectations, pharma
companies can propose ideas of “win-win”
activities to be carried out through engagements
The benefits the KOLs will draw in terms of
personal awareness and competence
development through the engagement should be
emphasized:
– Opportunity to participate in publication of articles,
interviews in media, presentations during congresses,
lectures during medical meetings, etc.
– Provide expert opinion/guidance and/or…
– … opportunity to participate in clinical research (e.g.
clinical trials) or to carry out IITs
1
– Professional development through the access to recent
information, to high education programs
2
, by working in
new research/medical areas, etc.
What do KOLs want
through engagements?
What should pharma companies
propose to KOLs?
2
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
25
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL selection
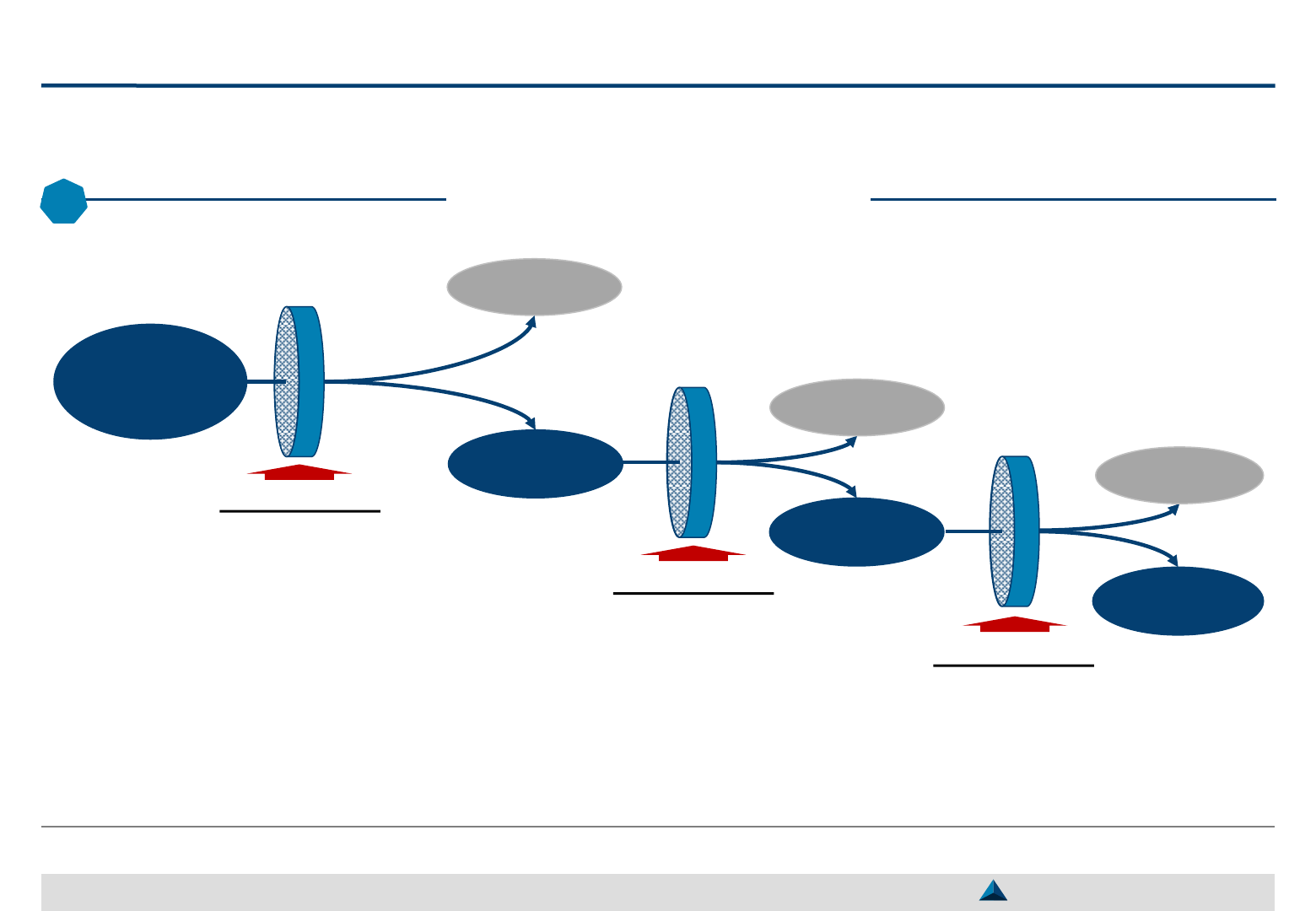
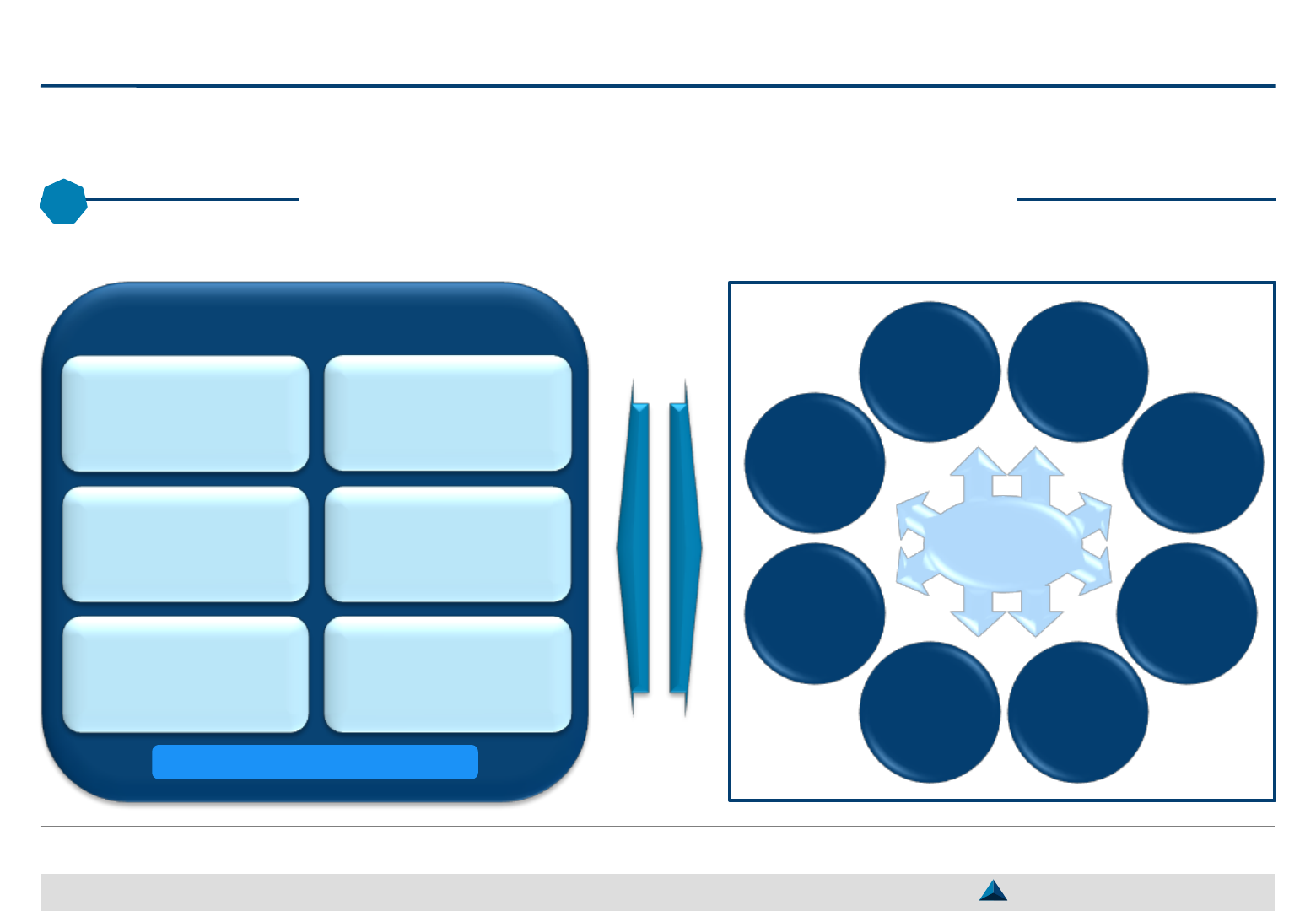
Pharma companies should be able to manage dynamically their selected KOLs by
attracting newcomers and putting an end to some existing collaborations
Dynamic management of selected KOLs
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
2
Current pool of selected KOLs
Guidance for new product development
Data generation (pre-clinical or clinical)
Creation of credible and persuasive medical content
Advice regarding product strategy (e.g. positioning)
Facilitation of patient access to new therapies
Entering KOLs Leaving KOLs
KOLs entering the reservoir of
partners should fulfill specific
objectives
Depending on the needs to be
fulfilled, the expertise and motives
of the KOL, the expected
engagement will be:
– Either strategic and renewed for
several years (partnership)
– Or tactic and carried out on an
ad-hoc basis (transaction) for a
specific activity (e.g. lecture,
clinical study)
To manage dynamically – and efficiently – a pool
of KOLs, it is important to stick to certain rules:
– The objective of the collaboration should be
clearly set to avoid any misunderstanding
– The expected engagement from the KOL and
services from the pharma company should be
specifically defined
– The fulfilment of the contractual obligations
should be closely monitored and the gaps, if
any, filled up by mutual agreement
KOLs may leave the reservoir of
partners on the basis of a:
– Joint decision (e.g. completion
of an ad-hoc agreement)
– Decision made by the pharma
company (e.g. engagement not
satisfactorily fulfilled, difficulty to
collaborate with the KOL)
– Decision made by the KOL (e.g.
mismanagement of the
relationship by the company,
lack of interest in the product or
the requested activities)
26
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement
1
Access limited to KOLs –
2
Each KOL should have a dedicated KOL Manager (e.g. a MSL) –
3
Continuous Medical Education –
4
Such as lectures to sales forces, face-to-face meetings
with the marketing team, etc. –
5
Such as visual aids, leaflets for patients
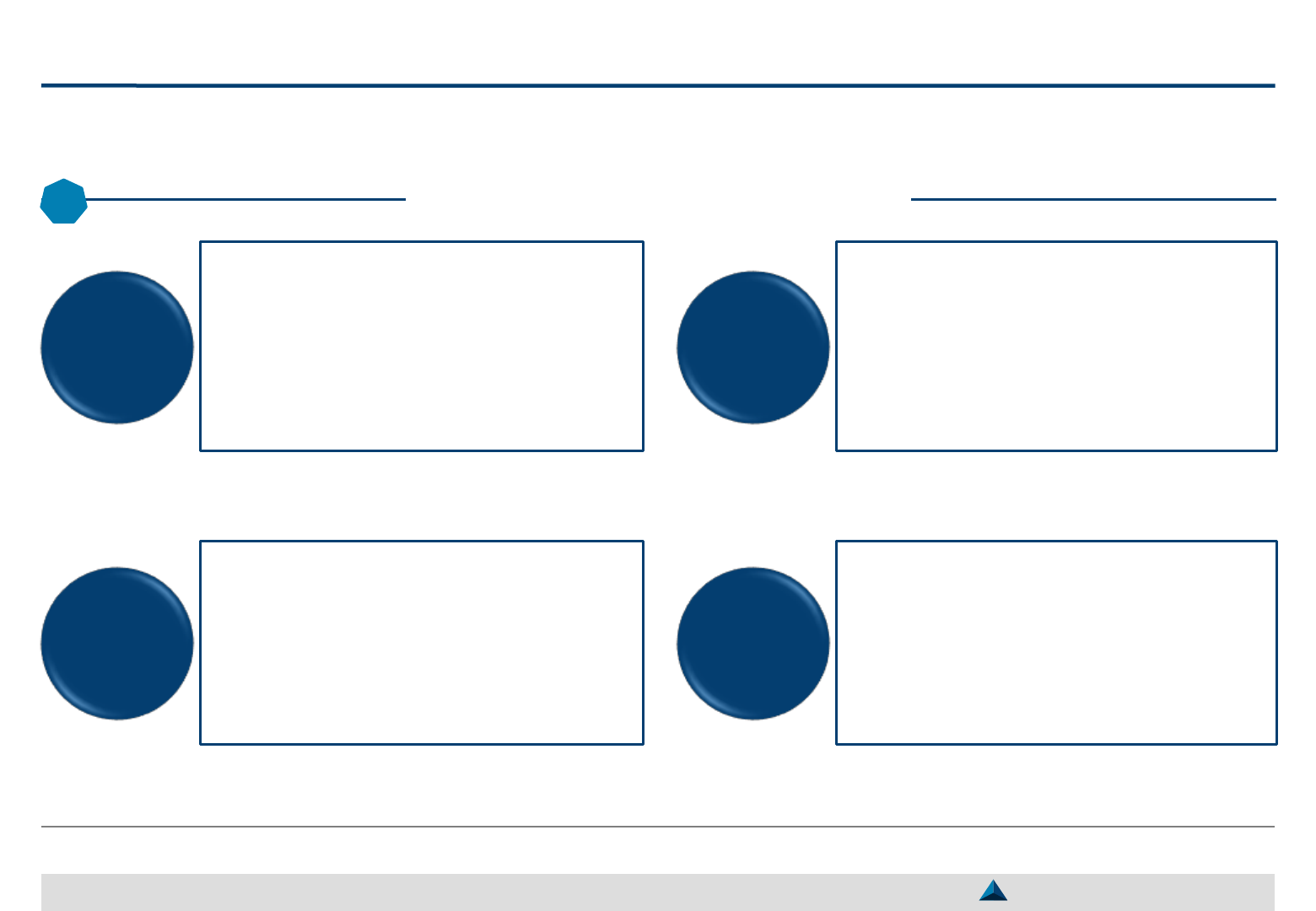
Pharma companies should balance what they expect from KOLs in terms of activities
and what they give them in terms of services to ensure a win-win partnership
Services proposed to & activities carried out by KOLs
27
May 2019
Activities carried out by KOLs (Illustrative) Services proposed to KOLs (Illustrative)
Lectures
during
symposia
Press
conference
Promo
material
review
5
Advisory
board
member
Participation
to internal
meetings
4
Training
of peers /
CME
3
Participation
to scientific
studies
Article
writing
KOLs
KOL Digital Platform (2.0)
1
Access to scientific
information
(e.g. articles, databases,
expert reports, clinical cases)
Ad hoc support
on demand basis
(e.g. media training, training
on statistics, change
management in a ward)
Organization of peer
meetings with top
international KOLs
(e.g. congresses,
symposiums, forums, etc.)
Slide kits for
training/teaching
programs
Technical support
to publish articles
(e.g. medical writing, proof
reading, peer pre-review)
Technical & funding
support for Investigator
Initiated Studies
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
KOL Manager
2
3
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement
1
Continuous Medical Education –
2
Physicians,
pharmacists, nurses, etc.
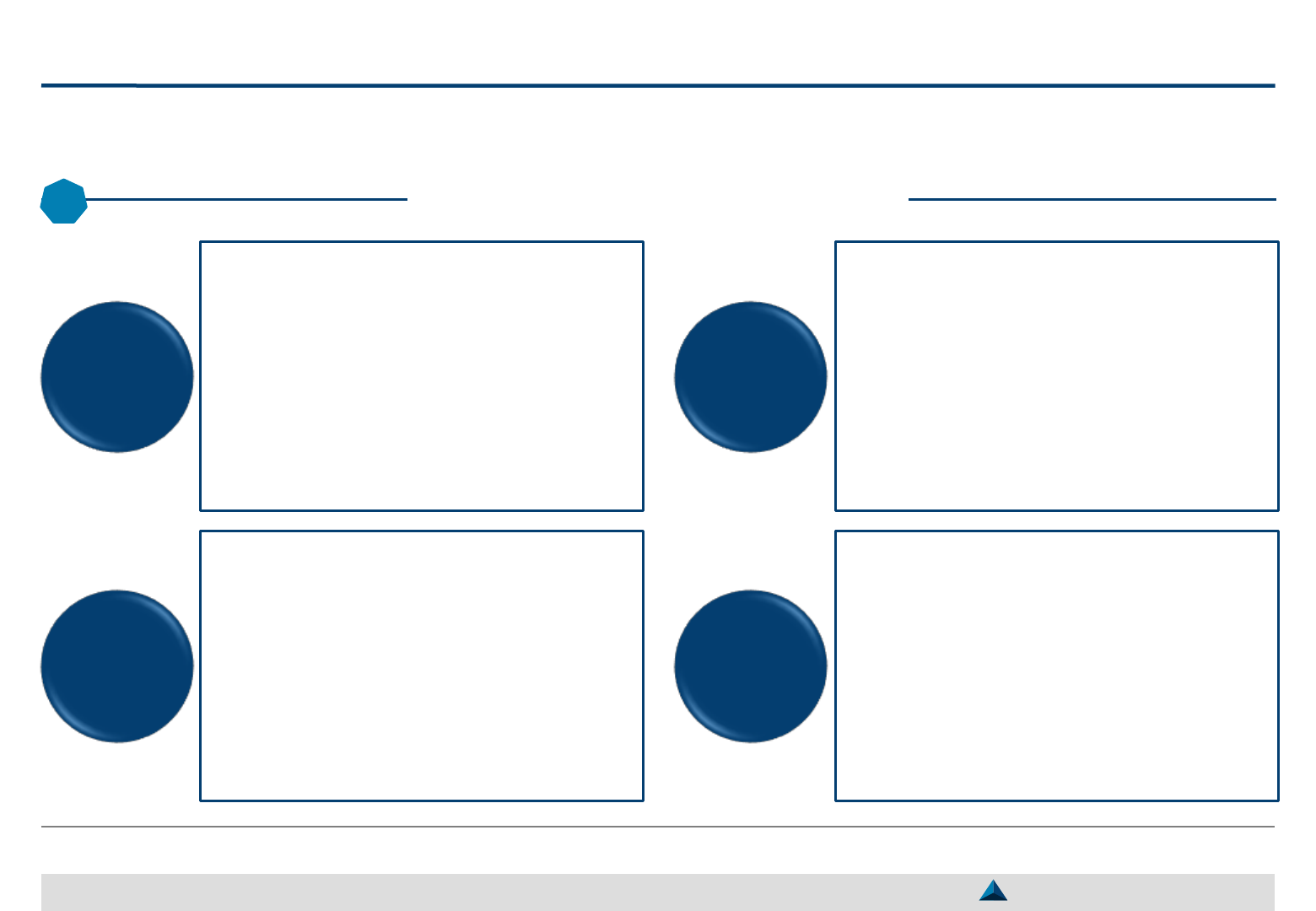
If KOLs share the objective of the pharma company and accept to communicate, the
following means can influence medical practices and help better position products
Potential value of KOL activities (1/2)
28
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
3
KOLs may support the pharma company
priorities by communicating in scientific
journals, professional magazines or lay
press regarding:
– New medical approaches, new guidelines,
patient management, etc.
– The position of its products in the therapeutic
strategy
Press conferences enable to have indirectly
access to a larger number of readers
The messages conveyed by KOLs may
sometimes be modified by journalists
It is rare for KOLs to make strong
statements in favor of a product during a
press conference
While giving lectures, KOLs may accept to
cover topics of interest for the company…
… and/or to position its product vs. direct
competitors or indirect therapeutic
alternatives based on scientific data/
rationale
KOLs may also share their own experience
as a prescriber of the company products
KOLs may communicate to HCPs during
training sessions regarding:
– Medical topics of interest for the pharma
company
– The position of its products in the
therapeutic strategy
In such circumstances, KOLs may convey
strong messages, if they decide to do so
H: Higher – M : Medium: – L: Lower
Lectures
during
symposia
Training
of peers /
CME
1
Article
writing
Press
conference
Perceived reliability by readers: H
Number of exposed readers: L-H
Perceived reliability by participants: M
Number of exposed attendants: L
Perceived reliability by readers: M
Number of exposed readers: M-H
Perceived reliability by participants: M-H
Number of exposed attendants: M
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement
KOLs can be of great value through direct collaboration (by training, informing,
giving advice, etc.) with medical and marketing teams of the pharma company
Potential value of KOL activities (2/2)
29
May 2019
KOLs may play an effective role during
internal meetings by:
– Informing / training medico-marketing teams
about scientific trends and position of
competitors
– Being invited as a “guest star” to show
collaborators the ability of the pharma
company to partner with top medical leaders
– Playing a role with sales reps (e.g. selling
forums)
Advisory board meetings with KOLs should
be preferred to individual meetings with
KOLs when the objective is to get advice on:
– Estimating the impact of key market trends:
• Scientific innovation
• New product development
• Evidence generation
• Market access strategy
• Marketing strategy (positioning)
– New ideas or concepts
KOLs may collaborate with the marketing
team by contributing to the creation of
promotional materials
Thus, they can create value by:
– Suggesting messages
– Developing a scientific rationale to support
messages/claims of the products
– Assessing and editing the content of
promotional materials (visual aid, booklet…)
KOLs, especially if they are supposed to
sign or co-sign the corresponding
publication, may be very helpful to:
– Participate to the design of the study
– Carry out the study (either about a given
pathology only or a pathology & its treatments
involving the pharmaceutical company
product)
Involvement of KOLs in medical/clinical
studies will depend on their field of interest
Promo
material
review
Participation
to scientific
studies
Advisory
board
member
Participation
to internal
meetings
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
3
Smart Pharma Consulting
Sources: Pharma's Guide to Effective KOL Engagement, Phanish Chandra (August 2017) –
Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement
A comprehensive KOL engagement strategy requires from pharma companies to
gain an in-depth understanding of KOL challenges, motivators and expectations
KOLs challenges – motivators – expectations
30
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
3
Expectations from pharma companies
Fair market value remuneration
Presence in KOLs field of expertise
Consistency, communication, support and interaction
Value-adding interactions with pharma companies collaborators
Research assistance
Credibility and commitment to patient care
Continuous engagement
Genuine involvement & meaningful partnerships
Transparency
Motivators
Prestige and renown
Better healthcare outcomes
Scientific journals and publications
Membership in advisory boards, steering committees
Formulation of guidelines and medical policies
Speaking opportunities at congresses, symposia
Participation in clinical trials and academic researches
Challenges
Trusting pharma: product efficacy and safety, corporate
reputation and service quality
Pharma engagement approach: transactional arrangement vs.
real relationship, multiple contact points
Time and doctor/patient ratio
Regulation: compliance, accountability, disclosure of
compensation from pharma companies
“One goal that most
KOLs share is to
capture attention and
prestige within their
community”
Smart Pharma Consulting
Sources: Study carried out in the UK, Uptake Strategies (January 2014) –
Smart Pharma Consulting analyses
2. Strategic KOL Engagement Planning KOL engagement
In general, the most common criticisms by KOLs at pharma companies are related to
absence of true partnerships and of cohesive internal strategy and processes
Top 10 poor pharma companies practices & key learnings
31
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
3
Top 10 poor practices
1. “30-page confidentiality agreement”
2. Unclear unspoken objectives
3. Inconsistent honoraria payments across projects
4. Strong commercial bias in discussions about treatments
5. Lack of listening
6. Lack of on-going communication
7. Sporadic approach: “No follow-up to show how they
used our input or what they did”
8. “17 different people from the same company contacted
me in the course of one month”
9. Changes in staff: “I never know who is who”
10. Relationship held by the CRO
Key learnings
Set clear objectives
Favor partnership-based to
transactional agreements
Consider what KOLs want from a
relationship with pharma companies
Ensure a transparent communication
Have a clear demarcation between
commercial, medical and clinical needs
(and others, if needed)
Ensure a consistent and coordinated
communication between the pharma
company and the KOLs
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement
The development of a KOL Engagement Plan is a centerpiece to maximize the
probability of success while partnering with KOLs
KOL engagement plan (1/2)
32
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
3
The development of a clear – precise – concise and shared engagement
(activity) plan, between KOLs and pharma companies – will ensure that:
– Objectives of collaboration are well understood and agreed upon
– Reciprocal expectations are well defined and accepted
– Respective commitments are fulfilled and in due time
The preparation of an engagement plan increases the probability of
success of the partnership over time…
… and minimizes the risks of mutual disappointments
The KOL Engagement Plan (KEP) will facilitate the coordination and the
communication across the pharma company and thus optimize synergies
across market access, medical and marketing departments
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement
1
If allowed by national and corporate regulations –
2
It is recommended to assign one KOL manager
who is the preferred point-of-contact for the KOL –
3
Ideally, twice a year –
4
Ideally, once a year
To build a useful and effective KOL Engagement Plan, it is recommended to follow
the 5-step process proposed here-below
KOL engagement plan (2/2)
33
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
3
1. Design of templates that can be shared
with KOLs and the pharma company
collaborators (i.e. from market access,
medical, marketing departments)
2. Filling up of the plan by the pharma
company collaborators assigned to the KOL
under the supervision of the Medical Director
and Marketing Director
1
3. Reviewing/adjustment of the plan by the
KOL and the KOL Manager
2
:
– Objectives
– Services offered by the pharma company
– Activities carried out by the KOL
– Fees (if any) at a fair market value
– Monitoring process of services/activities
5. Assessment of the engagement by:
– The KOL Manager and the KOL to
measure the level of mutual satisfaction
and decide about potential adjustments
3
– A committee incl.: the Medical Director,
the Marketing Director, the KOL Manager
to evaluate the KOL engagement and
decide about potential adjustments
4
4. Follow-up of the plan:
– Prepare the planned services/activities
– Analyze the quality of execution of these services/activities
– Reconsider – if not anymore relevant – planned services/activities
“To find common ground is a key success factor in KOL engagement”
Smart Pharma Consulting
Sources: Smart Pharma Consulting
Individual KOL Engagement Plans should be co-developed by the KOL and the
pharma company to avoid any misunderstanding and subsequent disappointments
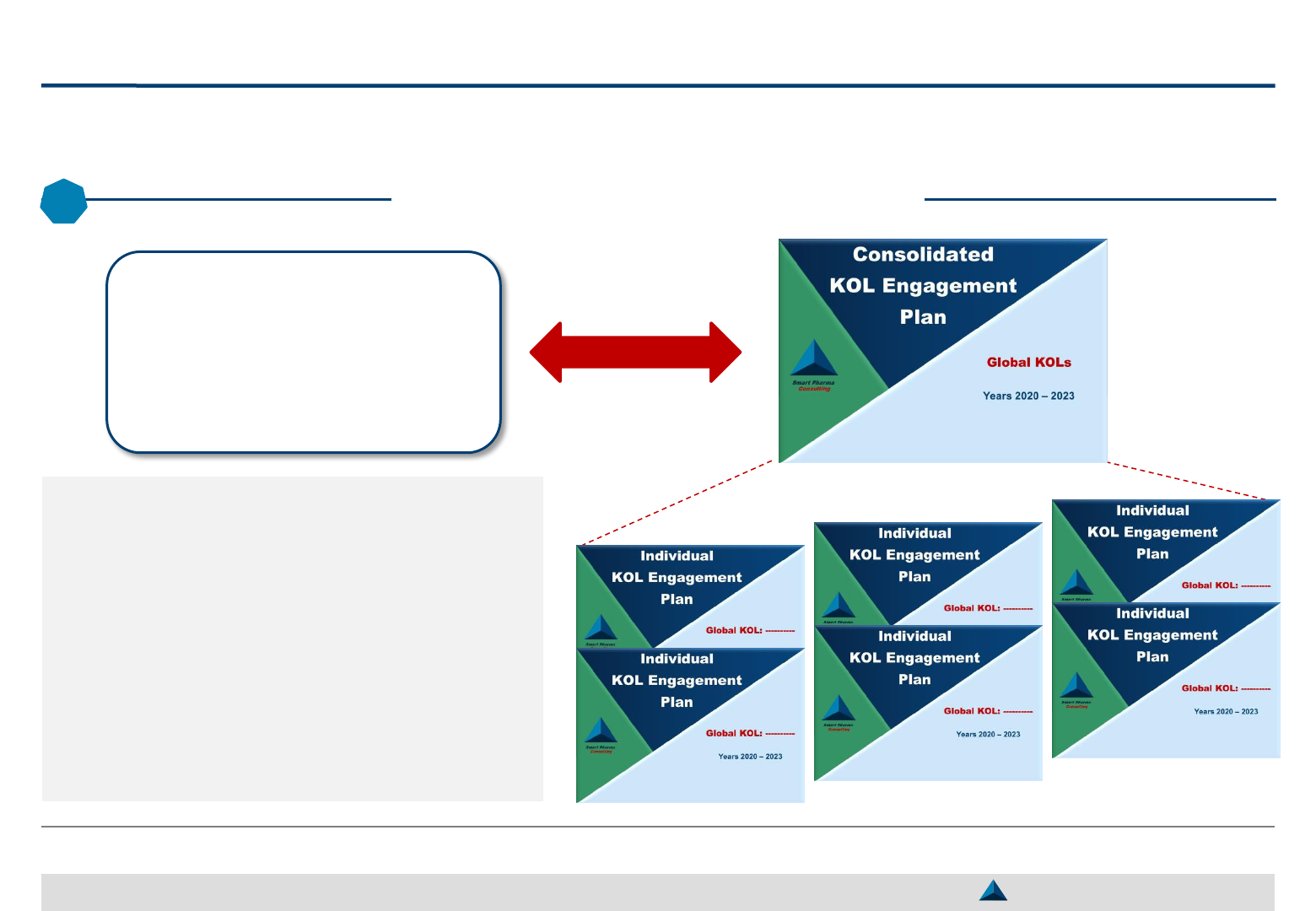
Development of KOL Engagement Plans
34
May 2019 Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Strategic Brand Plan
(2020 – 2023)
3
The KOL engagement plan should be
developed to support the Brand Strategic
Objective as per the Strategic Brand Plan
Each individual KOL engagement plan
should be designed accordingly and be
consolidated in a single document
The Consolidated KOL Engagement Plan
can cover a period lasting from one year to
3 or even 5 years, depending on the
product position on its life cycle
2. Strategic KOL Engagement Planning KOL engagement
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement
1
Key performance indicators –
2
Key execution indicators
35
May 2019
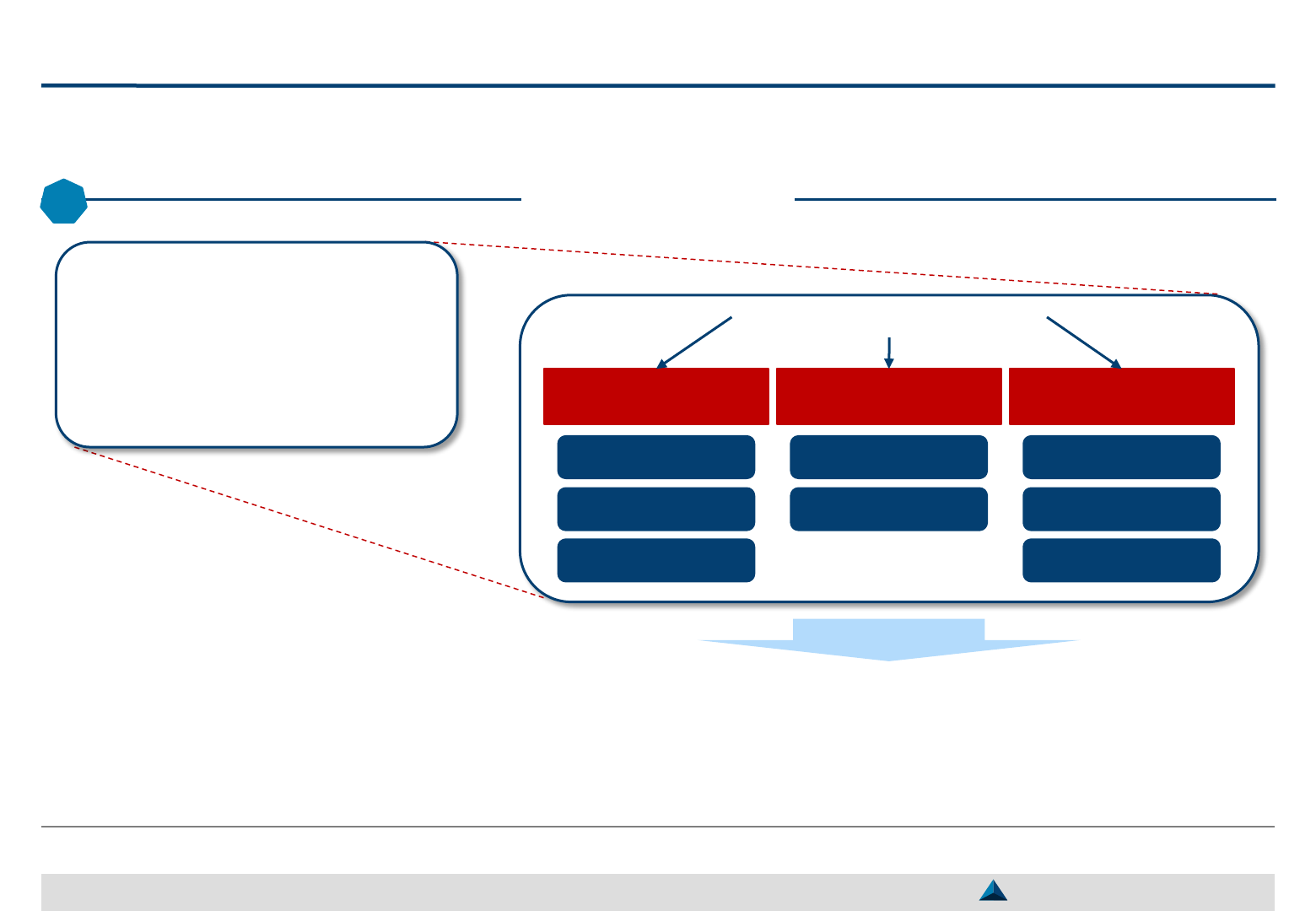
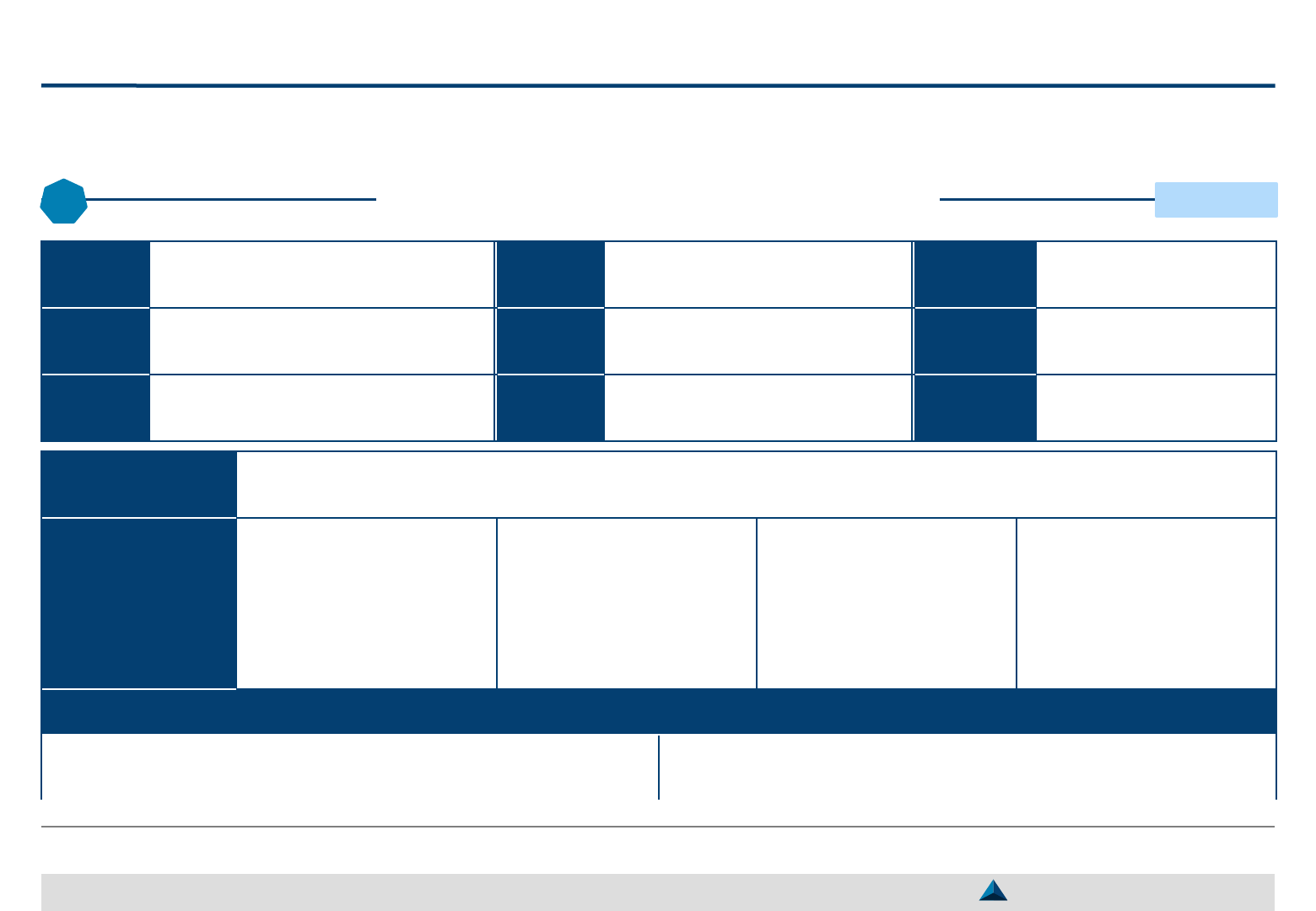
Structure of a Consolidated KOL engagement plan
The KOL Engagement Plan should be formalized in a document that could be
structured as proposed in the table of contents, here-below
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
3
Table of Contents
Introduction
– Brand Strategic objective (vision)
– Brand Strategic Imperatives & Critical Success Factors
– Brand development priorities (3-year perspective)
Expected contribution from the pool of Global KOLs
Expected contribution from individual Global KOLs
– Type of agreement (ad-hoc, partnership, duration, etc.)
– Key activity selection (e.g. advisory board meeting,
lecture, clinical study, peer-to-peer trainings)
– Key activity description (e.g. objective, timing,
accountability, budget)
– Key activity monitoring (e.g. KPIs
1
and KEIs
2
)
Illustrative
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement
1
Examples: Development of a digital tool to improve patients adherence, coordination of a multi-centric study, expert support
to estimate the medico-economic value of a new product, lectures during medical meetings organized with peers, etc.
36
May 2019
KOL
name
First name – surname
Medical
status
MD – head of medical department –
professor of medicine, etc.
Medical
setting
Private hospital –
Public hospital –
Teaching hospital
Expertise
E.g. therapeutic area, organ, pharmacology,
academic and/or clinical research,
scientific advisory boards, etc.
Awareness
Publications – Lectures –
Communication skills - Network
Impact
Index
1
Numerical scale to be
determined
Degree of
Interest
Low – Moderate – High
Points of
vigilance
E.g. mobility, adherence to deadlines,
quality of presentation documents, etc.
Ranking
Primary objectives
of the collaboration
•
Specific activities
planned within the
engagement
1
• • • •
Type of agreement Duration of the agreement
• Transactional agreement:
• Partnership agreement:
• Annual: from: ---/---/--- to: ---/---/---
• Multi-year: from: ---/---/--- to: ---/---/---
Individual KOL engagement plan – ID Card
The KOL Engagement Plan should include key information extracted from the KOL
database, specify the objectives of the collaboration, its scope and duration
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
3
Illustrative

Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement & monitoring
37
May 2019
Individual KOL Engagement Plan – KOL Activity Card
The KOL Engagement Plan should describe the activities the KOL is engaged to
carry out to meet specific objectives, and it should include monitoring indicators
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
3
Illustrative
Key implementation steps Timing Points of caution
Expected output / value
of the activity for…
• •
… the KOL
herself/himself
… the pharma
company
… 3
rd
parties
-------------------
• •
• • •
• •
• •
• •
Feasibility (High – Moderate – Low) Key Execution Indicators Key Performance Indicators
Technical •
• These indicators measure the quality of
execution of the activity
• These indicators measure the impact
(output/value/benefit) of the activity for
the different targets (the KOL, the
pharma company and possibly for 3
rd
parties, like peers, patients, PAGs)
Regulatory •
Financial •
KOL Activity
• Lecture, training of peers, advisory board,
press conference, article writing, IIS,
clinical study, etc.
Objectives
•
Pharma
company
contact
point
4

Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement & monitoring
38
May 2019
Individual KOL Engagement Plan – Partnership-based Service Card
The KOL Engagement Plan should also describe, plan and follow up the services
proposed to the KOL, as a constituent of the partnership-based agreement signed
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
3
Illustrative
Key implementation steps Timing Points of caution
Expected output / value
of the service for…
• •
… the KOL
herself/himself
… the pharma
company
• •
• •
• •
• •
• •
Feasibility (High – Moderate – Low) Key Execution Indicators Key Performance Indicators
Technical •
• These indicators measure the quality of
execution of the service provided to the KOL
• These indicators measure the impact of
the service provided to the KOL
Regulatory •
Financial •
Pharma
company
services
• Access to scientific information, technical
support to publish articles, provision of
training/teaching materials, organization
of peer meetings, etc.
Objectives
•
Pharma
company
contact
point
4
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement & monitoring
1
Logistics, timing, actual costs vs. budget –
2
Collects and collates disparate information on the online
activity surrounding scholarly content
Key execution and performance indicators are essential to optimize the chance of a
proper execution of services / activities and of a win-win partnership
Examples of tools to monitor engagements with KOLs (1/2)
39
May 2019
KOLs activities Key execution indicators (KEIs)
Key performance indicators (KPIs)
Lecture during symposia
or congresses
Interest (10-point scale)
Utility (10-point scale)
Practicality (10-point scale)
Implementation
1
(10-point scale)
Global level of satisfaction of attendees (10-
point scale)
Inclination of attendees to support & prescribe
the product:
– Number of lectures/trainings/publications
–
Quality/objectivity of messages conveyed to peers,
pharmacists, PAGs, etc.
Training of peers
Article writing
Acceptance by recognized
journals (scientific, medical, or in
lay press, etc.)
Post on highly regarded websites
Impact factor and Altmetrics
2
(for scientific /
medical journals)
Number of broadcasted issues for lay press
Number of views / likes on Internet
Contribution of content to support
the product
Press conference
Number and quality of press
conferences conducted
Participation in scientific
studies
Implementation (number of
patients recruited, timing, actual
costs vs. budget)
Publication of an article in a renowned
scientific journal
Impact of the publication on product
reputation
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
4
Smart Pharma Consulting
Sources: Smart Pharma Consulting
2. Strategic KOL Engagement Planning KOL engagement & monitoring
1
Investigator Initiated Trails –
2
Logistics, timing, cost vs. plan
Key execution and performance indicators are essential to optimize the chance of a
proper execution of services / activities and of a win-win partnership
Examples of tools to monitor engagements with KOLs (2/2)
40
May 2019
Pharma company services
Key execution indicators (KEIs) Key performance indicators (KPIs)
Access to scientific
information
Interest (10-point scale)
Utility (10-point scale)
Practicality (10-point scale)
Implementation
2
(10-point scale)
Global level of satisfaction of KOLs
(10-point scale)
Inclination of KOLs to support the
pharma company products:
– Number of lectures / trainings /
publications
– Quality/objectivity of messages
conveyed to peers, pharmacists,
patients, etc.
Increased level of KOLs awareness
and reputation
Increased level of products
awareness
and reputation
Organization of peer
meetings with top global /
international KOLs
Publications support
IIT
1
support
Slide kits for training /
teaching programs
Ad hoc support on demand
basis
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
4
Smart Pharma Consulting
Sources: Study carried out in the UK, Uptake Strategies (January 2014) –
Smart Pharma Consulting analyses
3. Conclusions
41
May 2019
Fewer opportunities for transactional and agreements (e.g. ad-hoc contributions such as lecture
at a symposium)
Greater independence of KOLs and increasing pro-bono contribution where mutual benefits lie
(e.g. research program, lectures reinforcing their awareness)
More independent collaboration projects, indirectly or not connected to a specific product (e.g.
research program, education program, best practice sharing)
Increasing presence, awareness and influence of KOLs on Internet
Broader definition of KOLs from clinical expert to patient advocate, payor, academic institution,
charity, etc.
Evolving internal policies to foster transparency and compliance with industry code of practice
Future trends
in KOL Engagement Planning
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Smart Pharma Consulting
Sources: Smart Pharma Consulting
3. Conclusions
42
May 2019
1. Define clear and precise objectives for each KOL
2. Build a relationship based on an exchange of services / activities (vs. fee-for-service deal)
3. Make sure that services provided to KOLs contribute to fulfill their needs/expectations
4. Ensure an open and transparent relationship
5. Do not ask KOLs to promote your products, you would affect their reputation and yours
6. Make the best use of KOLs limited time by organizing useful exchanges
7. Assign a KOL Manager who is the KOL-preferred contact point and who ensures alignment and
information sharing between all collaborators of your company in contact with her/him
8. Create a technology platform to store, structure and share data relative to KOL profiles and
engagements (planned and achieved)
Define internal guidelines and a control process to prevent
any compliance issues that could damage your corporate reputation
Recommendations
for a Successful KOL Engagement Planning
Strategic KOL Engagement Planning for a better Efficacy & Efficiency
Smart Pharma Consulting
Consulting firm dedicated to the pharmaceutical sector operating
in the complementary domains of strategy, management and organization
Smart Pharma Consulting
Smart Pharma Consulting Editions
Besides our consulting activities which take
85% of our time, we are strongly engaged in
sharing our knowledge and thoughts
through:
– Our teaching and training activities
– The publication of articles, booklets,
books and expert reports
As of today, more than 100 publications in free access can
be downloaded from our website
Since the beginning of 2019, we have published one
business report (The French Pharma Market 2018 – 2023)
Since the beginning of 2018, we have published:
– 10 position papers in the “Best-in-Class Series” and 3 in
the “Smart Manager Series”
Our research activities in pharma business management and
our consulting activities have shown to be highly synergistic
We hope that this new publication will interest you and we
remain at your disposal to carry out consulting projects or
training seminars to help you improve your performance
Best regards
Jean-Michel Peny
Best-in-Class Series
This series intends to share concepts, methods and tools to
boost the efficiency and efficacy of executives having
operational responsibilities in the pharma business
We have yet published eight Best-in-Class issues:
Strategic KOL Engagement Planning
This position paper proposes an approach and a selection of
enabling tools to help pharma companies effectively and
efficiently engage with KOLs (Key Opinion Leaders)
We recommend an approach in four steps:
1. Objective setting
2. KOL selection
1. MSLs
2. Pharma Marketers
3. Medical Reps
4. Hospital KAMs
5. Pharma BD&L
6. Pharma Market Research
7. Pharma Strategy Crafting
8. Pharma Field Force Organization
9. Hospital & Institution
Relationships in Regions
1, rue Houdart de Lamotte – 75015 Paris – France • Tel.: +33 6 11 96 33 78 • E-mail: jmpeny@smart-pharma.com • Website: www.smart-pharma.com
3. KOL engagement
4. KOL monitoring
