
Corporate Presentation
AstraZeneca Pharma India
Limited (AZPIL)
May 07, 2013

2
Disclaimer
2
This presentation by AstraZeneca Pharma India Limited (the “Company”) is solely for your information and may not be taken away, distributed, reproduced, or
redistributed or passed on, directly or indirectly, to any other person (whether within or outside your organization or firm) or published in whole or in part, for any
purpose by recipients directly or indirectly to any other person. By accessing this presentation, you are agreeing to be bound by the trailing restrictions.
This presentation does not constitute an offer or invitation to purchase or subscribe for any securities in any jurisdiction, including the United States. No part of it
should form the basis of or be relied upon in connection with any investment decision or any contract or commitment to purchase or subscribe for any securities.
None of our securities may be offered or sold in the United States without registration under the U.S. Securities Act of 1933, as amended. This presentation is not
intended to be a prospectus (as defined under the Companies Act, 1956) or an offer document under the Securities and Exchange Board of India (Issue of Capital
and Disclosure Requirements) Regulations, 2009 as amended.
Further, in order, among other things, to utilise the 'safe harbour' provisions of the US Private Securities Litigation Reform Act 1995, we are specifically providing the
following cautionary statement: This presentation may contain certain statements with respect to the operations, performance and financial condition of the
Company and/or AstraZeneca Plc, which may be construed as forward-looking statements. Although we believe our expectations are based on reasonable
assumptions, any forward-looking statements, by their very nature, involve risks and uncertainties and may be influenced by factors that could cause actual
outcomes and results to be materially different from those predicted. The forward looking statements, if any, reflect knowledge and information available at the date
of preparation of this presentation and the Company undertakes no obligation to update these forward-looking statements. Important factors that could cause actual
results to differ materially from those contained in forward-looking statements, if any, certain of which may be beyond our control, include, among other things: the
loss or expiration of patents, marketing exclusivity or trademarks, or the risk of failure to obtain patent protection; the risk of substantial adverse litigation/government
investigation claims and insufficient insurance coverage; exchange rate fluctuations; the risk that R&D will not yield new products that achieve commercial success;
the risk that strategic alliances and acquisitions will be unsuccessful; the impact of competition, price controls and price reductions; taxation risks; the risk of
substantial product liability claims; the impact of any failure by third parties to supply materials or services; the risk of failure to manage a crisis; the risk of delay to
new product launches; the difficulties of obtaining and maintaining regulatory approvals for products; the risk of failure to observe ongoing regulatory oversight; the
risk that new products do not perform as we expect; the risk of environmental liabilities; the risks associated with conducting business in emerging markets; the risk
of reputational damage; the risk of product counterfeiting; the risk of failure to successfully implement planned cost reduction measures through productivity
initiatives and restructuring programmes; the risk that regulatory approval processes for biosimilars could have an adverse effect on future commercial prospects;
and the impact of increasing implementation and enforcement of more stringent anti-bribery and anti-corruption legislation. Nothing in this presentation should be
construed as a profit forecast.
This presentation is for general information purposes only, without regard to any specific objectives, financial situations or informational needs of any particular
person. No representation or warranty, express or implied, is made as to, and no reliance should be placed on, the fairness, accuracy, completeness or correctness
of the information or opinions which may be contained in this presentation. Such information and opinions are in all events not current after the date of this
presentation. The Company may alter, modify or otherwise change in any manner the content of this presentation, without obligation to notify any person of such
change or changes.

3
Overview
3
1
2
3
AstraZeneca Plc Overview
AZPIL: Business Overview
AZPIL: Key Highlights
4
Overview
4
1 AstraZeneca Plc Overview

5
AstraZeneca Plc: Business Overview
5
Global research and innovation driven Integrated
biopharmaceutical Company focusing on the discovery,
development & commercialization of prescription
medicines in 3 core therapeutic areas namely Cardio-
metabolism, Oncology and Respiratory & Inflammation,
and also present in the therapeutic areas of
Neuroscience and Infection & Vaccines, on an
opportunity-driven basis
Formed in April 1999 through the merger of Astra AB of
Sweden and Zeneca Group Plc of UK
Ranks among the top 10 pharmaceutical companies
globally with CY2012 revenues of $ 27.97 bn and
CY2012 core operating profits of $ 10.43 bn, with a
market capitalization of $ 64.91 bn (as on May 03, 2013)
Sixth fastest growing MNC pharmaceutical company
across emerging markets (Emerging Markets revenue
was up 9% in Q1CY13 vis-a-vis Q1CY12 (CER))
Operations in more than 100 nations globally and
employs around 51,700 employees worldwide
Global Sales (Geographical Split)
Strong Brand Portfolio
8 brands with Sales of more than $1billion in 2012
Source: AstraZeneca Plc, Annual Reports and Investor
Presentations
6
AstraZeneca Plc: Vision & Identified Growth
Platforms
6
To be a global biopharmaceutical business delivering great medicines to patients through innovative science
and excellence in development and commercialization
A science-led, innovation strategy
Broad R&D platform focused on 3 core Therapeutic Areas (TAs)
Balanced portfolio of specialty and primary care products
Global commercial presence, with strength in emerging markets
Vision
Identified Growth Platforms
1. Cardiovascular / Brilinta
2. Diabetes
3. Emerging Markets
4. Respiratory
5. Japan
Source: AstraZeneca Plc, Annual Reports and Investor Presentations
AstraZeneca Plc: Focus on distinctive science
in 3 core therapy areas
7
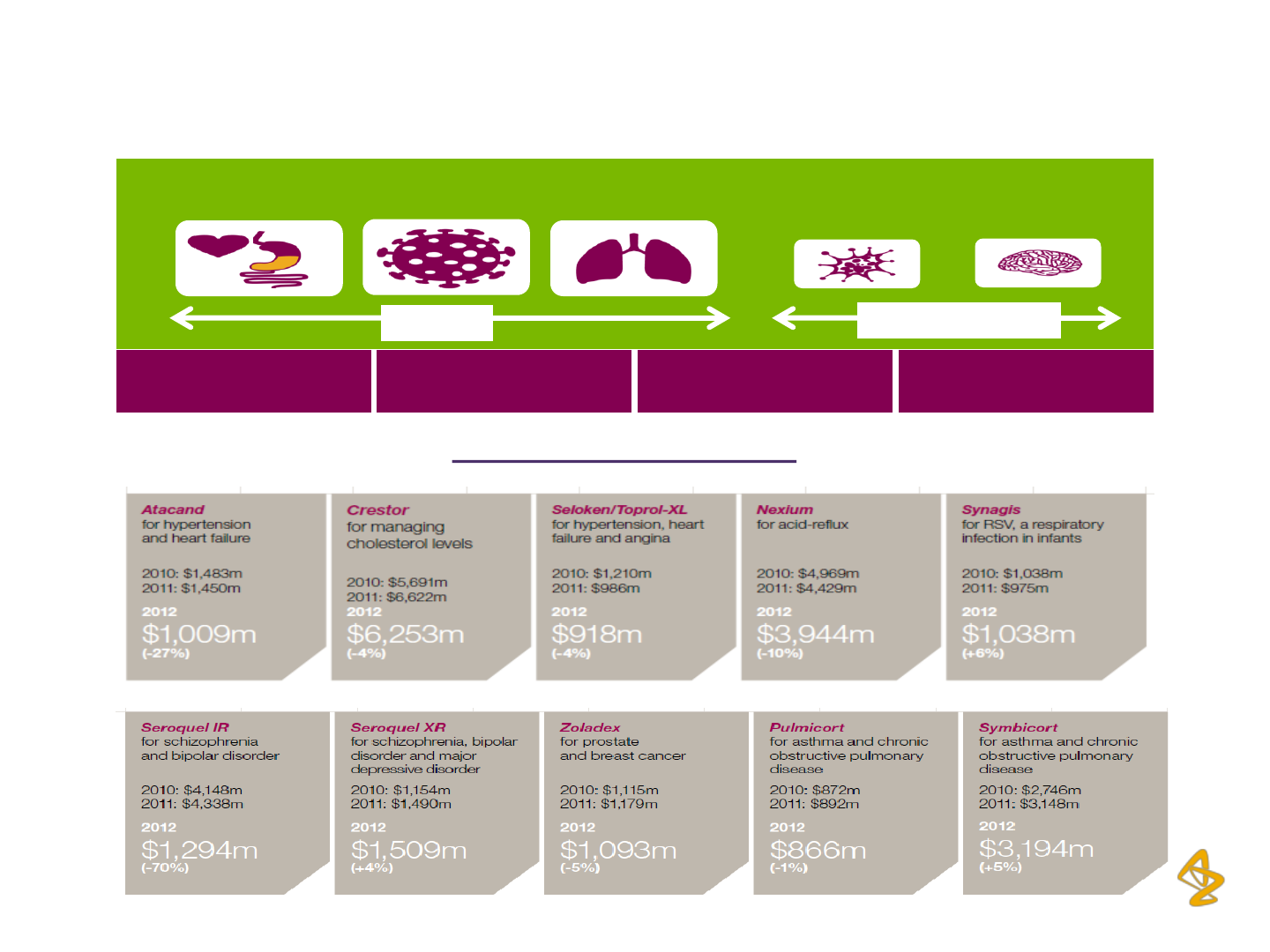
Key Products and Brands
Cardiovascular
Neuroscience
Gastrointestinal Infection
Respiratory & Inflammation Oncology
Neuroscience
Infection &
Vaccines
Cardio- Metabolism
Respiratory/
inflammation
Core TAs
Oncology
Opportunity-Driven
Protein
engineering
Biologics
Small
Molecules
Immuno-therapies
Source: AstraZeneca Plc, Annual Reports and Investor Presentations

8
AstraZeneca Plc: R&D Overview
Over $4.5 billion investment in R&D in CY2012, with
nearly 9,800 employees working in R&D Division
R&D presence in 10 principal R&D centres in six
countries (including one in India), covering both small
molecules and biologics
Strong and growing R&D presence in Asia
R&D efforts focused on three key therapy areas: Cardio-
metabolism, Oncology and Respiratory & Inflammation;
opportunistic R&D investment in Infection & Vaccines
and Neuroscience
Collaborations with different companies globally which
has further augmented the current R&D pipeline, with
approximately 40% being sourced externally
Setting up strategic research and development centers
in the U.K., U.S. and Sweden to improve pipeline
productivity and to aide in establishing AstraZeneca as a
global leader in biopharmaceutical innovation
Key Strategic Centers for R&D activities
Gaithersburg
Primary location for
Company's biologics
activities, and Global
Medicines;
Development activities
for small and large
molecules
Global centre for
research and
development, with a
primary focus on small
molecules
Mölndal
Cambridge
Note: These three strategic sites will be supported by other
existing AstraZeneca facilities around the world, including
Boston, Massachusetts, US which will continue to be a centre
for research and development, with a primary focus on small
molecules
Set up of New site in
Cambridge with close
proximity to University of
Cambridge and world
class UK Bioscience
community.
Source: AstraZeneca Plc, Annual Reports and Investor Presentations
Phase I
26 NMEs
Phase II
21 NMEs
Phase III / Registration
6 NMEs
moxetumomab*
MEDI0639*
AZD2014
MEDI3617* AZD1208
MEDI-565* AZD9150
volitinib*
MEDI6469*
MEDI4736*
AZD8330*
MEDI4212
AZD8848*
AZD7594*
AZD5363*
AZD3293*
ATM AVI
AZD1446*
MEDI2070*
MEDI9929*
MEDI5872*
MEDI5117
MEDI-557
MEDI-559
MEDI-550
MEDI-551*
tremelimumab
AZD4547
MEDI-573* selumetinib*
benralizumab* AZD5069
Olaparib
#
mavrilimumab*
MEDI8968*
AZD2115*
sifalimumab* AZD1722*
tralokinumab
AZD6765
AZD5423*
MEDI7183*
AZD3241
AZD5847
AZD5213
brodalumab* lesinurad
metreleptin*
naloxegol*
fostamatinib*
CAZ AVI*
Legend
Oncology
R&I
CVMD
Neuroscience
Infection
Large Molecules Small Molecules Large Molecules Small Molecules Large Molecules Small Molecules
AZD7624
MEDI-546*
Changes since FY2012: MEDI-575 and MEDI7814 discontinued; AZD3480 returned to Targacept; AZD7624 progressed into Phase I; and
AZD1722 progressed into Phase II.
Note: CXL status is pending an FDA discussion.
Parallel indications not shown above: fostamatinib (haematological malignancies); MEDI-551 (multiple sclerosis); and tralokinumab
(ulcerative colitis).
* Partnered product
AstraZeneca Plc: Robust R&D Pipeline…
10
Source: AstraZeneca Plc, Annual Reports and Investor Presentations
#
Decision to accelerate Olaparib to Phase III,
and committed to EU filing in 2013
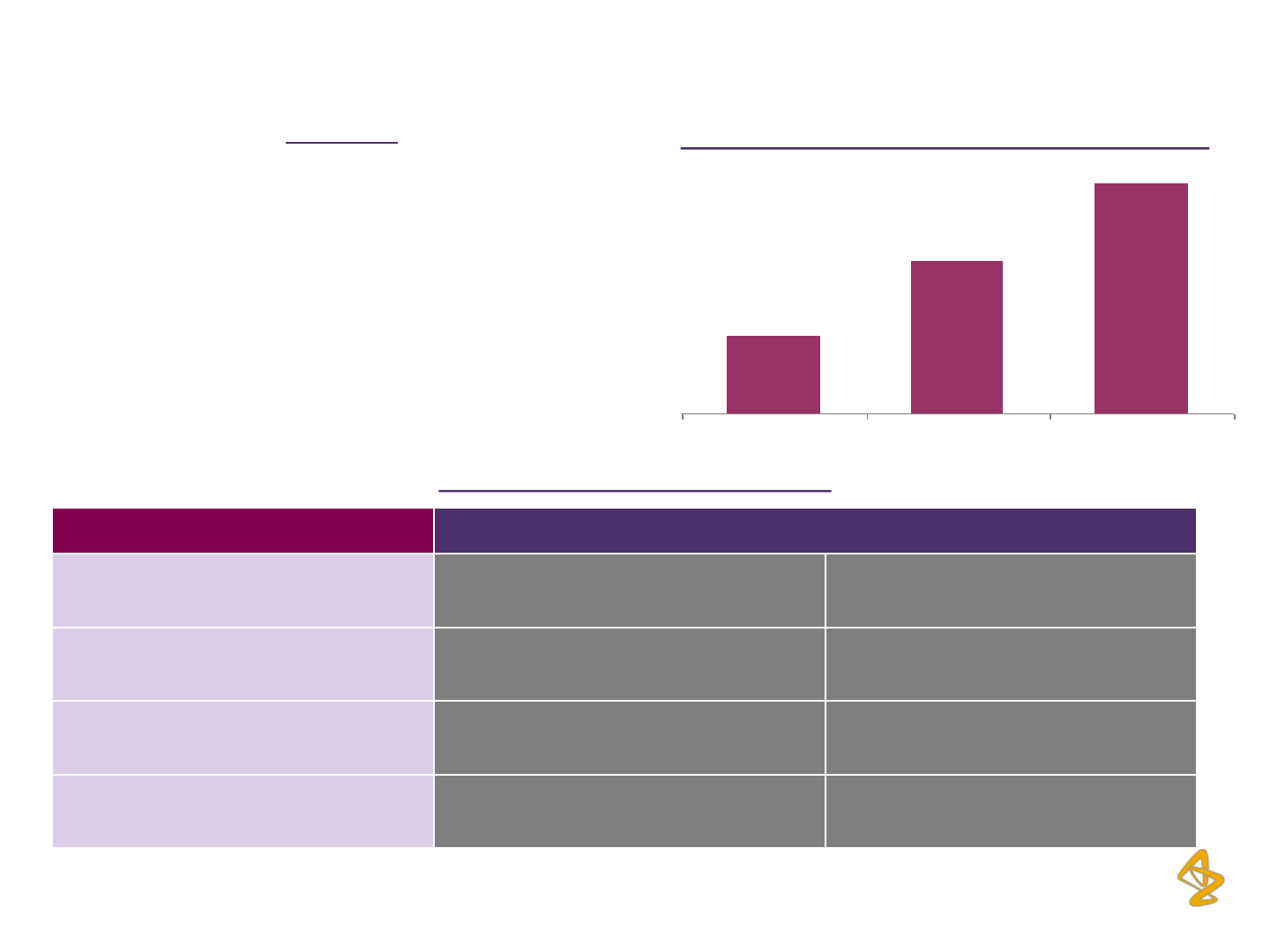
2012 2013E 2016E
11
…With Impressive Phase III Portfolio…
11
Anticipate ~5-7 NME Phase III Starts
2013 2014
benralizumab
asthma
AZD6765
depression
ATM AVI
serious infections
Olaparib
#
solid tumours
sifalimumab/MEDI-546
systemic lupus erythematosus
AZD4547
gastric cancer
moxetumomab pasudotox
hairy cell leukaemia
mavrilimumab
rheumatoid arthritis
AZD5069
asthma
selumetinib
non-small cell lung cancer
MEDI-551
haematological malignancies
tralokinumab
asthma
In 2013 – 2014, AstraZeneca Plc anticipates ~5 – 7
NME Phase III starts
10 potential NME submission opportunities between
now and 2016
By 2016, AstraZeneca Plc will reach its target
volume in Phase III and Registrations
Phase III & Registration NME pipeline volume (#)
Near Term
6
8
9 - 10
Source: AstraZeneca Plc, Annual Reports and Investor Presentations
#
Decision to accelerate Olaparib to Phase III, and committed to EU filing in 2013
12
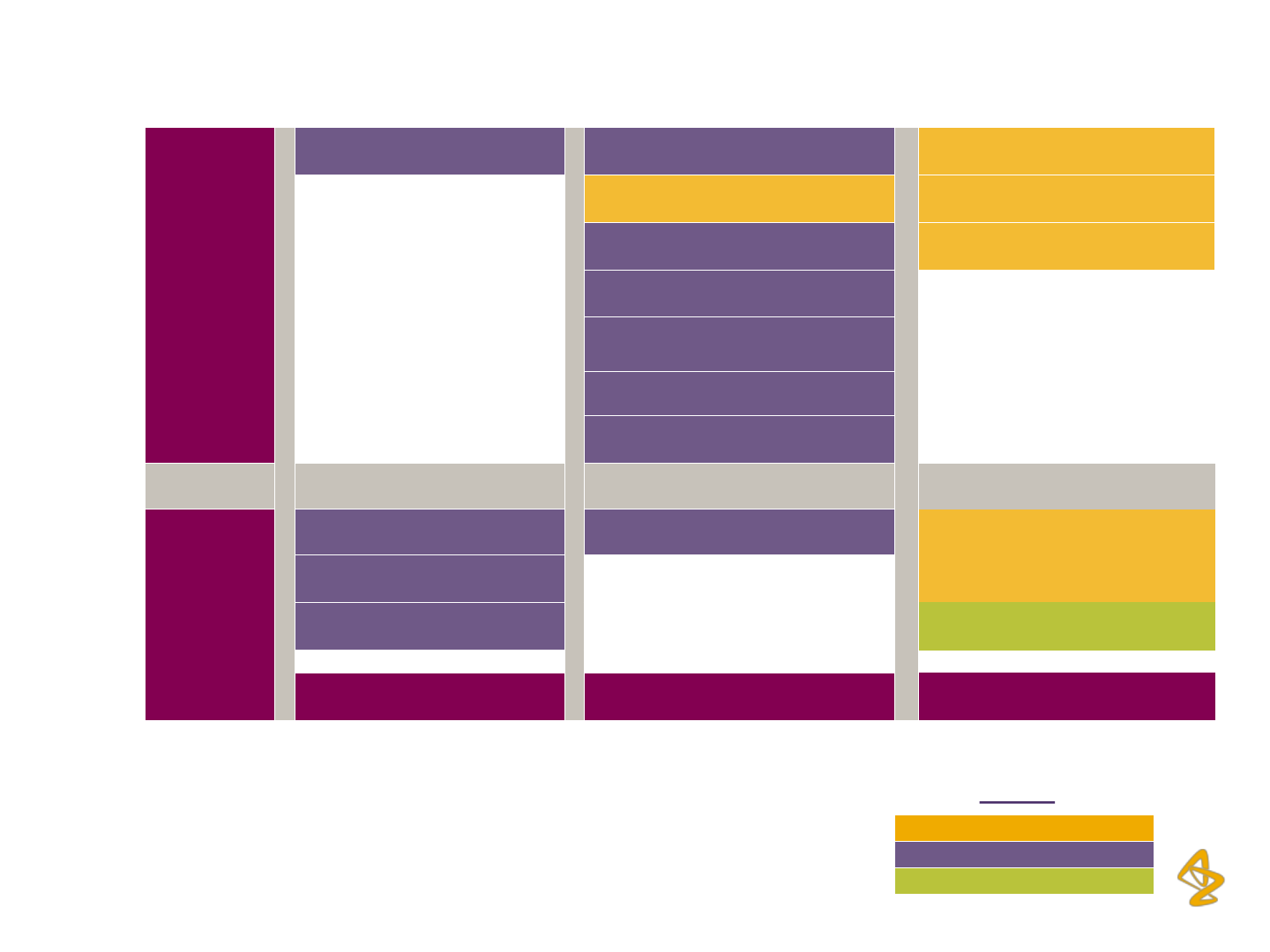
…Providing Attractive growth opportunities
Over
$ 1Bn
AZD5069 benralizumab Brodalumab^ (2015)
fostamatinib (2013) lesinurad (2014)
MEDI-551 naloxegol (2013)
Olaparib
#
(2013)
selumetinib
sifalimumab / MEDI-546
tralokinumab
Upto
$ 1Bn
AZD4547 ATM AVI CAZ AVI (2014)
AZD6765 metreleptin (2013)
mavrilimumab Moxetumomab
Low Medium
High
Phase III
Phase II
Phase I
Legend
Potential peak year sales for
New Medicines
KEY: (20xx) Year in brackets represents planned year of regulatory submission
^Gross revenue – not AZ share for Brodalumab
Peak Year Sales (PYS) includes lifecycle management opportunities
Strength of evidence to date
Source: AstraZeneca Plc, Annual Reports and Investor Presentations
#
Decision to accelerate Olaparib to Phase III,
and committed to EU filing in 2013
13
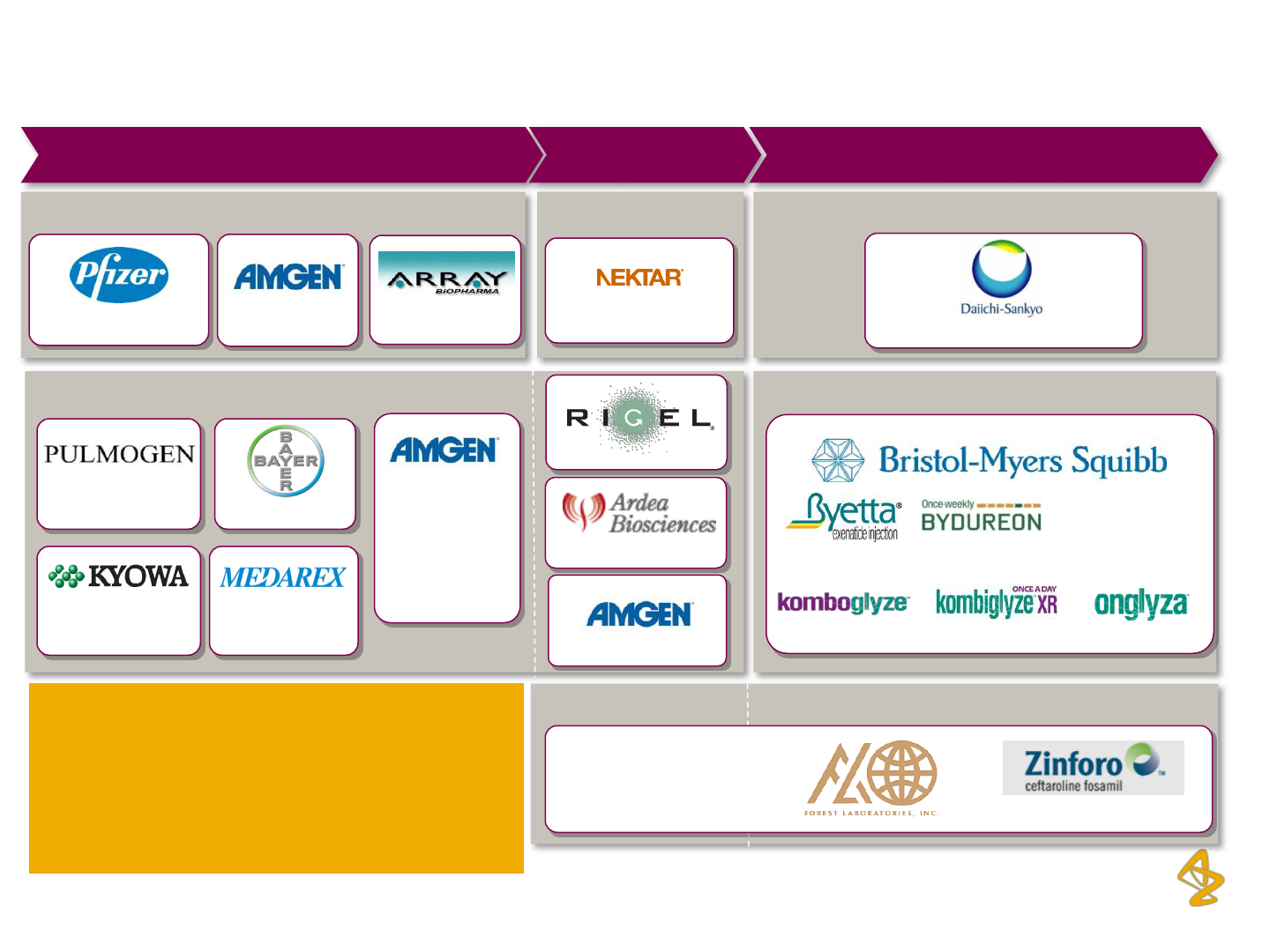
AstraZeneca Plc: Expanding partnership pipeline
13
Oncology CNS
Diabetes
Infection
Respiratory & Inflammation
Oncology
Benralizumab
Lesinurad
Fostamatinib
AZD5423
Tremelimumab
Phase II
Phase III/
Registration
Launched/
Approved (2012)
Selumetinib
AZD2115
AMG139
AMG157
AMG181
AMG557
MEDI-8968
Sifalimumab
MEDI-546
RANMARK (Japan)
CAZ AVI
MEDI-575
Brodalumab
Naloxegol
EU
FORXIGA
Source: AstraZeneca Plc, Annual Reports and Investor Presentations
AstraZeneca Plc has also recently entered
into an exclusive development agreement
with Moderna Therapeutics, collaboration
with Karolinska Institutet and announced
the acquisition of AlphaCore Pharma
14
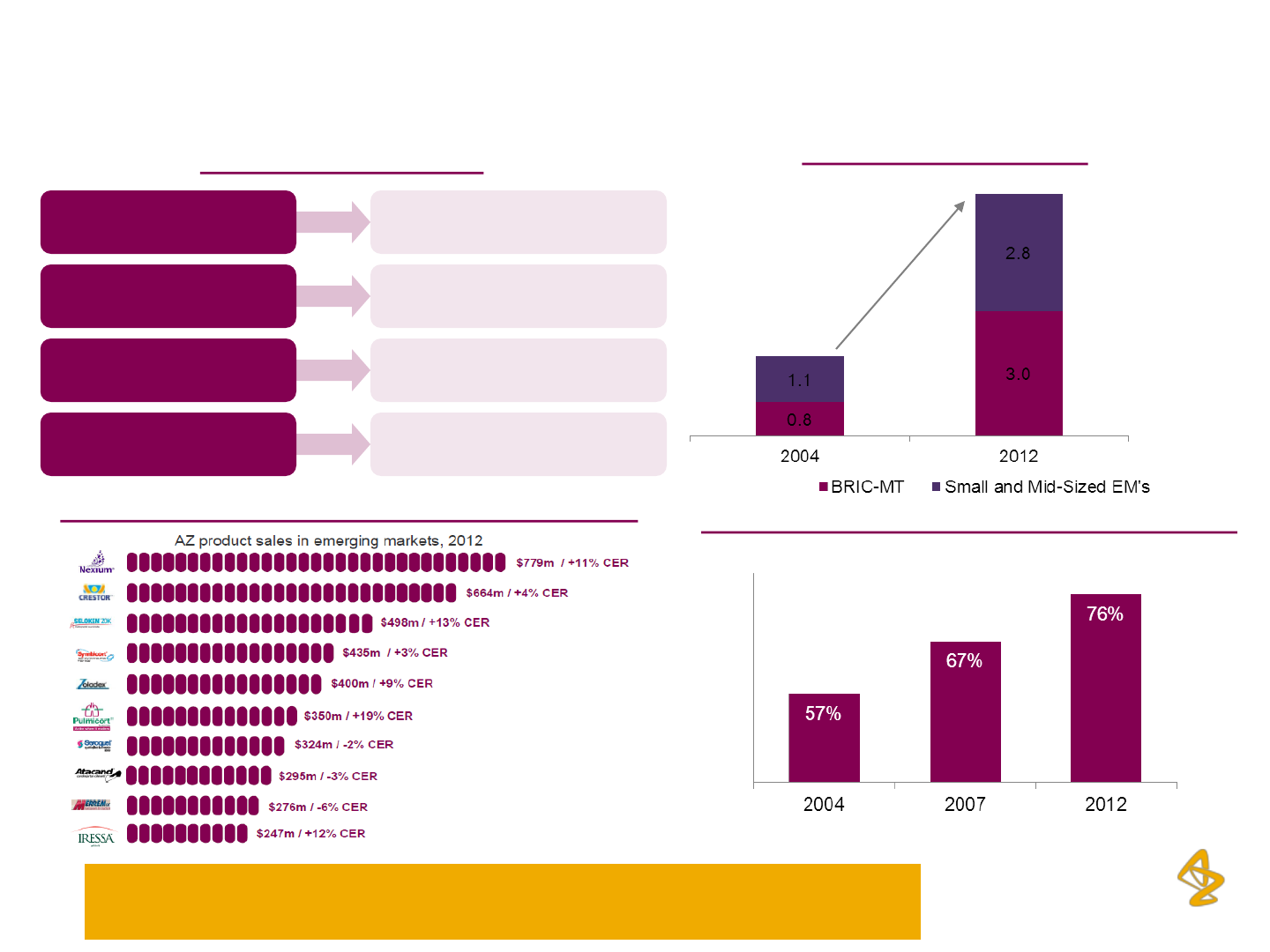
Focus on Emerging markets– A platform for
sustained growth
Emerging Markets Growth
$ Billion
$ 1.9 bn
$ 5.8 bn
Absolute
Growth
CAGR
$ 3.9 bn
$1.7 bn
$2.2 bn
15%
12%
18%
Successful Portfolio of Brands across EM’s (in $ mn)
Emerging Market Strategy
AstraZeneca is targeting high single digit growth in Emerging markets
through to 2016, with special focus on the top 15 markets, including India
Improving profitability* across emerging markets
Company’s current emerging markets margins are
similar to its Europe business 7-8 years back
*pre-R&D emerging markets operating margin (excluding central
costs), indexed to 2012 margins in established markets
Invest Early in Key
Markets
Build Share of Voice with
Best-in-Class sales force
Develop strong local
leadership
Focus on AZ products and
build BGx business
Accelerate Investment in Top
15 markets
Expand reach with multi-
channel capabilities
Transform market access,
medical affairs & affordability
Refocus on AZ portfolio &
Innovative in-licensing
Source: AstraZeneca Plc, Annual
Reports and Investor Presentations
15 15
2 AZPIL: Business Overview
Overview
16
AZPIL: Business Overview
16
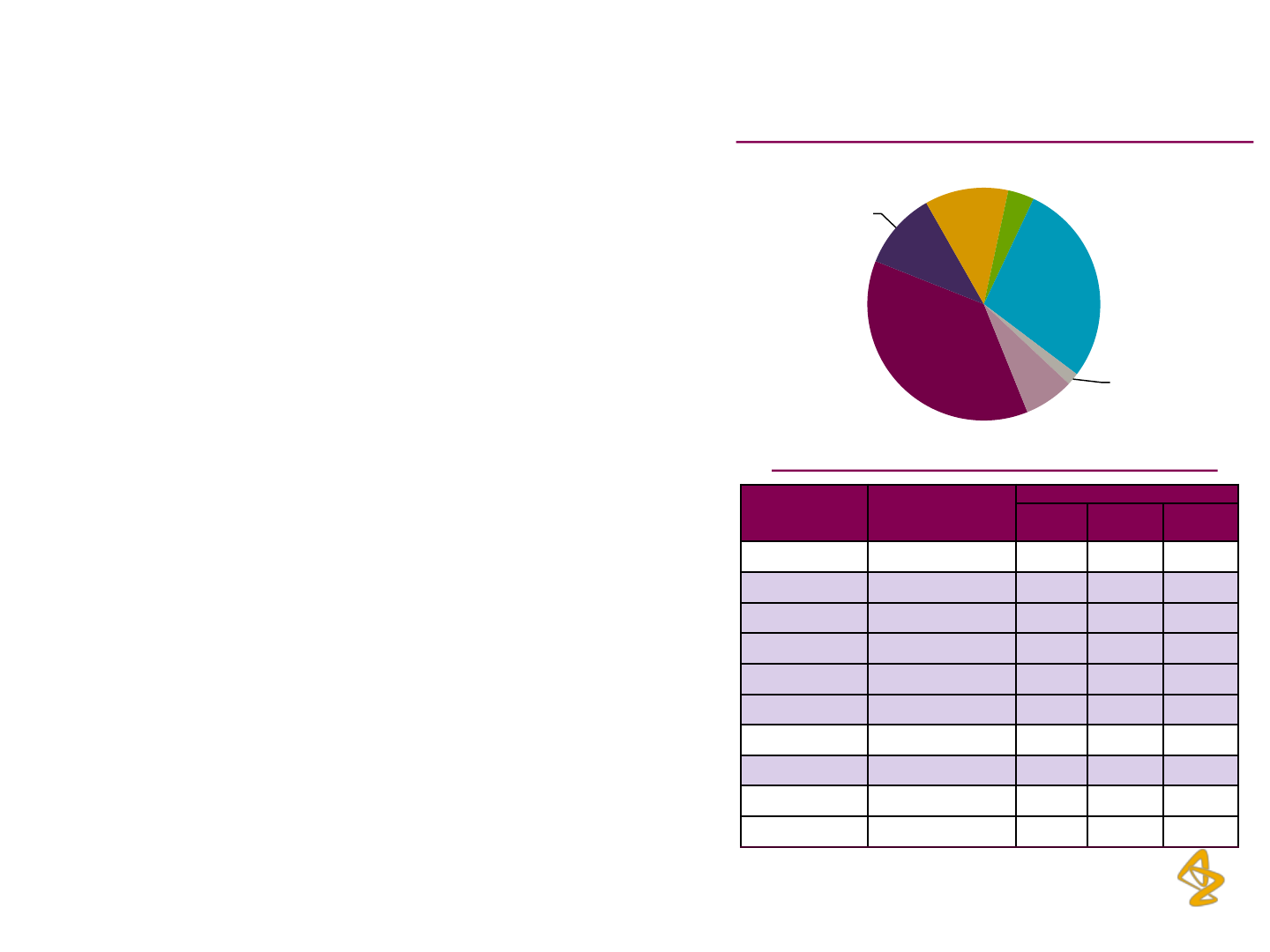
FY13 Therapeutic Area-Wise Sales Contribution (%)
AZPIL is a subsidiary of AstraZeneca Pharmaceuticals AB
Sweden (89.99% shareholding), which is in turn a wholly
owned subsidiary of AstraZeneca Plc, UK, and has been
present in India since 1979, with its corporate
headquarters located in Bengaluru, Karnataka
AZPIL is present in the therapeutic areas of Cardio-
metabolism, Oncology, Respiratory & Inflammation,
Infection, Local Anesthesia and Maternal Healthcare
AZPIL’s manufacturing facilities are spread across 64
acres at Bengaluru, and commenced commercial
production in 1982
AZPIL is currently setting-up a state-of-the-art tablet
manufacturing facility with a capacity of 1.2 billion tablets
per year at a cost of Rs. 1,005 million
AZPIL has a total employee strength of ~1,588 including
dedicated sales force of ~1,166 FTEs (March 31, 2013)
AZPIL has been regularly launching products from its
global portfolio in India over the past years, leading to the
development of several domestic power brands including
Crestor, Seloken XL, Meronem, Arimidex, Zoladex,
Neksium and most recently Brilinta
Brand Therapeutic Area
Total Sales (Rs. mn)
FY 11 FY 12 FY13
Meronem Anti-Infectives
769
819
764
Linctus Codeinae Respiratory
518
489
150
Seloken XL Cardiac
317
381
386
Xylocaine Pain / Analgesics
393
377
64
Betaloc Cardiac
345
352
234
Imdur Cardiac
324
339
195
Neksium Gastro Intestinal
162
212
235
Crestor Cardiac
155
212
230
Zoladex Oncology
135
137 162
Arimidex Oncology
122
130
123
AZPIL Top 10 Brands (Based on FY12 Sales)
Cardio,37%
Respiratory,11
%
Oncology,12%
Local
Anaesthesia,4
%
Infection,28%
Maternal
Health
Care,2%
Gastro,7%
Highlighted brands have been impacted on account of the voluntary
recall initiated by AZPIL in Q4FY12
17
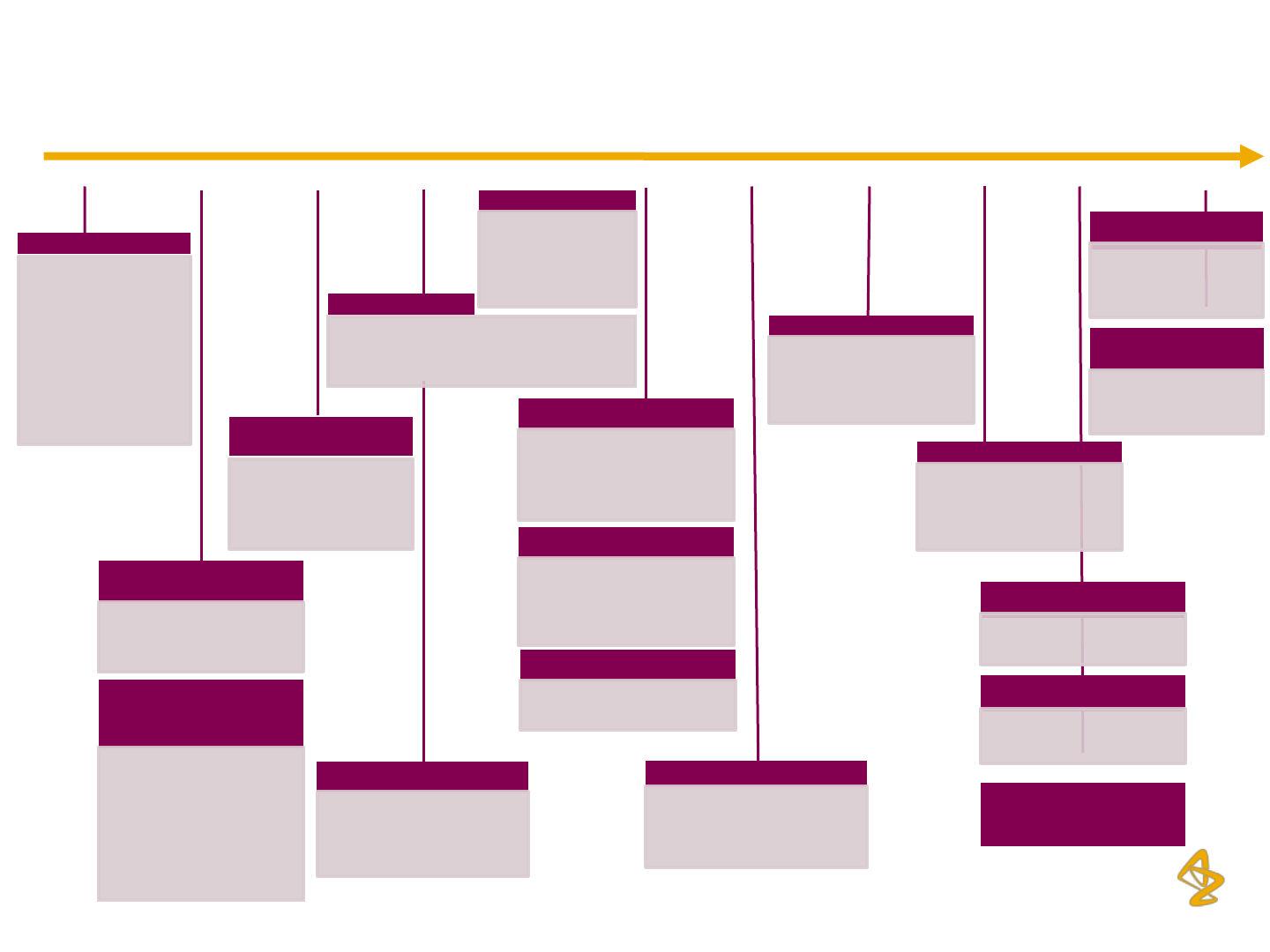
AZPIL: History & Key Milestones
17
2007 2001 2009
Start of Operations
Acquisition of majority
shareholding of AZPIL
by AstraZeneca
Pharmaceuticals AB
Sweden (AZAB)
(56.5%), following the
merger between Astra
and Zeneca
Introduction of Seloken
XL
Introduction of Seloken
XL in India from the
global portfolio
2006 2003 2002 2008
Introduction of Products
Introduced Symbicort,
(from the global portfolio)
and Gladis & Valencia
2004 2005
Open Offer for Acquisition
of further Shares
In May 2002, AZAB
increased its shareholding
to 87.21% through the first
open offer; In Dec 2002,
AZAB’s shareholding in
AZPIL reached 91.61%
through second open offer
API Facility Approval
API facility was approved by
MPA (Swedish Regulatory
Authority)
New Products
• Acquisition of Vancocin
from Eli Lily
• Launch of Partocin in
India
Introduction of Products
Introduced Faslodex & Iressa
from Global Portfolio and
Selomax & Clavatrol
Udaan Project Launched
Introduced Crestor & 7
Other Products under
Udaan Project
6 More Products Introduced
6 More Products launched
under Udaan Project
Introduction of Global
Brands
2010-11 2012
Introduction of Meronem &
Zoladex (from global
portfolio);
IT Systems
ERP system was
extended to Depots;
HRIMS system was
implemented
Awards
• No. 1 ranking in medical
rep survey
• Manufacturing excellence
award Frost & Sullivan
API Facility Approval
• Japanese FDA approval
for TBS
Brilinta
Brilinta launched in
India from the global
portfolio
Investment in New Tablet
Manufacturing Facility
Stake Reduction
Promoter reduced its stake in AZPIL from
91.61% to 89.99% over 2004 and 2005, to
comply with the local regulations
Launch of Onglyza
Launch of Kombiglyze
XR
Launch from Diabetes
Alliance Portfolio
Launch from Diabetes
Alliance Portfolio

18
AZPIL: Key Therapy Areas & Brands
18
Cardiovascular
•Brilinta^
•Onglyza*^
•Crestor^
•Seloken XL^
•Betaloc, Betaloc
H
$
•Imdur
$
•Ramace,
Ramace H
$
•Plendil
$
•Zestril
$
•Selomax
@
•Seloram
#
•Vigocil, Vigocil
M
#
•Nitract SR
•Valfect, Valfect
H
#
•Olways, Olways
H, Olways AM
#
•Kombiglyze XR*
Respiratory
•Symbicort^
•Bricanyl
$
•Mit’s Linctus
Codeinae Co
%
•Mit’s Linctus DX
%
•Bricarex,
Bricarex A
%
•Bricacef,
Bricacef PED
#
•Rhinofex
#
•Rhinomax
#
•Rhinocort
$
•Pulmicort
Respules
$
Maternal
Healthcare
•Prostodin
%
•Cerviprime
%
•Primiprost
%
•Partocin
@
•Gladis
@
•Valenzia
@
Oncology
•Zoladex ^
•Arimidex ^
•Nolvadex ^
•Iressa ^
•Casodex ^
•Faslodex ^
Infection
•Meronem^
•Vancocin CP
•Actamase
#
•Enclere
#
•Remergin
#
•Rescade
#
Neuroscience
•Diprivan^
•Xylocaine
$
•Sensorcaine
$
•Naropin
#
Gastrointestinal
•Neksium^
Leading Brands Across Therapeutic Areas
* In alliance with Bristol Myers Squibb
^ Global products introduced after 2001,
$
Global products introduced prior to 2001
#
BGx Udaan Products
%
AZPIL Local portfolio introduced prior to 2001
@
AZPIL Local products introduced after 2001 (but before Udaan Project)
Key Brand Highlights
Meronem has consistently been the No.1 brand in Carbapenems category and is
also the No. 1 Brand in the Hospital Market Segment as per IMS MAT Mar’13
Seloken XL has been the No.1 brand in the Metoprolol Once Daily market, since
last 5 years and has been growing faster than the category
Brilinta has been amongst the most successful product launches in the Indian
Pharmaceutical market, clocking sales of Rs. 51 million within 6 months of launch
Neksium has been growing faster than the gastro-intestinal therapeutic segment,
where it is present
Source: IMS Health

19
AZPIL: Manufacturing Facilities
19
Existing Formulation Facility Existing API Facility
New Tablet Manufacturing Facility -
Formulations
Capacity: 690 mn tablets
Commercial Production
Commencement : 1982
Location: Bengaluru
No. of Employees: 210
Capacity: 3600 Kgs
Commercial Production
Commencement : 1982
Location: Bengaluru
No. of Employees:37
Capacity: 1.2 billion tablets per year
Construction completed (Validation ongoing)
Location: Bengaluru
Expected Commencement of Commercial
Production: Q1FY14

AZPIL: Managing Director
Mr. Sanjay Murdeshwar
Sanjay, aged 46 years joined AZPIL as the Managing Director on May
02, 2013 and has over 17 years of diverse experience in the
Pharmaceutical Industry working across various roles and regions
with Bayer AG
Most recently, he was working as the Vice President and Head,
Commercial Operations for Bayer Healthcare – Pharmaceuticals for
Asia Pacific region. He was responsible for marketing, strategy
development, sales and marketing excellence, and business
development for the Company for Asia Pacific countries
Prior to this role, he was the Country Head – Bayer Healthcare India,
and Managing Director of Bayer Pharmaceuticals Private Limited
Previously, he has also held position for Country Head and General
Manager for Bayer Healthcare, Philippines
Graduate in Chemical Engineering from Mumbai University and has
completed Masters in Business Management from Asian Institute of
Management, Philippines
20

21
Mr. Robert Ian Haxton
Rob, aged 43 years joined AZPIL as the Whole Time Director and Vice
President – India Operations on February 01, 2013 and has over 20
years of diverse experience in the Pharmaceutical Industry working
across various roles and regions with AstraZeneca
Most recently, he was working as the Head of Regional External
Supply (EMEA), for AstraZeneca Plc. He managed a multicultural and
multi geographic team located in UK, Turkey, Russia, South Africa,
Egypt and Israel. He developed the 5 year category strategy for the
EMEA contractor supply base
Prior to this role, he was Supplier Account Manager – Global External
Sourcing, and Product Supply Chain Manager with AstraZeneca Plc
Previously, he has also been Plant Manager in factories across the
AstraZeneca Global network
Graduate (BSc. Hons) in Biomedical Technology from Sheffield
Hallam University
AZPIL: Whole Time Director & VP – India Operations
22
Mr. Justin Ooi
Non-Executive Director
Mr. Justin Ooi, aged 42 years has completed post graduate qualification at MGSM (Australia) and executive
programes at INSEAD and London Business School.
He has over 25 years of experience. He has been with AstraZeneca for over 19 years and has gained
comprehensive experience across both commercial and financial functions - with the last 10 years being part
of the Senior Executive Team. He is currently the Area Finance Director for AstraZeneca International Region
(including Asia and India). Prior to this role, he has held various senior level positions including Area Finance
Director for AstraZeneca Asia Pacific Region, and Sales & Marketing Director and Chief Financial Officer for
AstraZeneca Australia.
AZPIL: Non-Executive Directors (AZ Group)
Mr. Ian Brimicombe
Non-Executive Director
Mr. Ian Brimicombe aged 49 years, is a graduate from King’s College, London. He has exposure on audit, tax
and corporate finance at Coopers & Lybrand, London (now PricewaterhouseCoopers) from 1986, qualifying as
a Chartered Accountant and a Chartered Tax Adviser.
He has been with AstraZeneca since 1994 and has held various senior positions in Corporate Finance and
Taxation. From 2001, he has been Director of Group Tax, responsible for global tax operations and delivery of
AstraZeneca's group tax targets. Currently, he is the Vice President – Corporate Finance for AstraZeneca Plc.
He has been on the Board of AZPIL since September 2006
23
Mr. D. E. Udwadia, Chairman
Independent Director
Mr. K. S. Shah
Independent Director
Mr. D E Udwadia, aged 73 years, holds Degree in BA (Hons) and LLB and also holds Master’s Degree in
Political Science and History. He has over 48 years of active corporate law practice and wide-ranging
professional experience. He is an Advocate and a Solicitor by profession. He is a Solicitor and Advocate of
the Bombay High Court and a Solicitor of the Supreme Court of England. He is a Senior Partner of M/s.
Udwadia, Udeshi & Argus Partners, a reputed law firm.
Mr. Udwadia has been on the Board of AZPIL from inception and since September 2000, as Chairman of the
Board. He is also on the Board of several other reputed companies.
Mr. K S Shah, aged 72 years, is a Graduate in Commerce and a Fellow Member of the Institute of Chartered
Accountants and a Fellow Member of the Institute of Company Secretaries of India.
He has rich experience in industry including general management and administration. Prior to his appointment
in the Company, he was the Finance Director and Deputy Managing Director of May & Baker (I) Ltd. He has
been on the Board of AZPIL since November 2001, and was the Managing Director of Astra IDL from July
1988 to September 1991. He is also Chairman of the Audit Committee of the Board.
AZPIL: Independent Directors
Mr. Narayan K Seshadri
Independent Director
Narayan K Seshadri aged 56 years, is a Chartered Accountant by profession with over thirty years of
professional experience.
He is the founder of Tranzmute Capital & Management Private Limited. Earlier, he had founded Halcyon
Resources & Management that had partnered with a US investment management group. Prior to establishing
Halcyon, he was the Managing Partner at KPMG’s Business Advisory Services Practice and was also a
member on Andersen’s global CEO advisory council. He holds Board positions in many companies. He has
been on the Board of AZPIL since December 2012.
AZPIL: Key Event Update
In Q4FY12, AZPIL initiated a voluntary recall of twelve products following AZ
Worldwide Audit Group’s (WWAG) quality audit, on account of which it faced supply
issues in FY13, which are currently in the final stages of being remediated
24
Voluntary Recall initiated in
Q4FY12
• Voluntary recall was initiated for
twelve products manufactured at
Bengaluru plant, following the
AstraZeneca Worldwide Audit
Group’s (WWAG) quality audit
• As a precautionary measure,
production was also voluntarily &
temporarily suspended to review
manufacturing & quality practices
at the plant, and undertake
remedial measures
• This resulted in further impact on
other non-recalled products as
well, and led to losses in FY13
Remedial Measures
• 54 experienced personnel from
AstraZeneca Global were
seconded to India on short as well
as longer term assignments to
help address these manufacturing
/ quality issues
• Site Investment with Managerial,
leadership, process and
equipment changes
• Comprehensive training plan
delivered
• Recruitment of diploma level
education shop floor workmen
Present Situation
• Key impacted products like
Xylocaine (Injection), Prostodin,
Seloken XL and Sensorcaine
have already been re-introduced
in the market
• The manufacturing of the non
tablet impacted products has
been outsourced to select
contract manufacturers in India
• Efforts are in place to reintroduce
the remaining impacted products
and re-establish AZPIL’s market
position
25
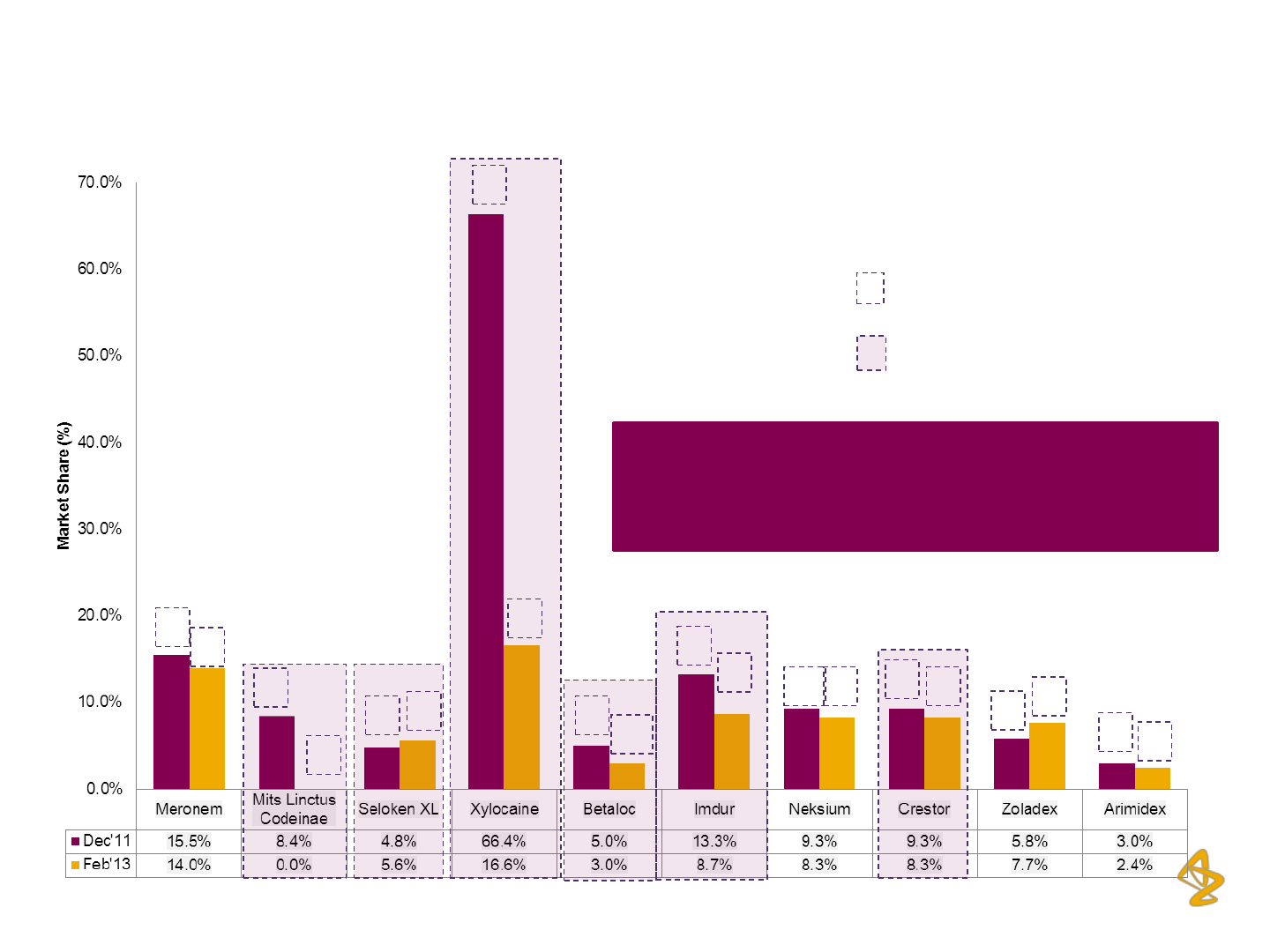
AZPIL: Non-Impacted Brands continue to deliver
Resilient Performance
Source: IMS Health
*Market Share based on MAT Values
Brand-wise Market Share
*
and Ranking
(Top 10 Brands)
1
1
3
6
3
3
1
2
2
10
2
3
11 9
3
3
4
3
6
9
Ranking
Brands Impacted due to Recall
6 out of the top 10 AZPIL brands rank amongst
the top 3 in their respective therapeutic
categories
26
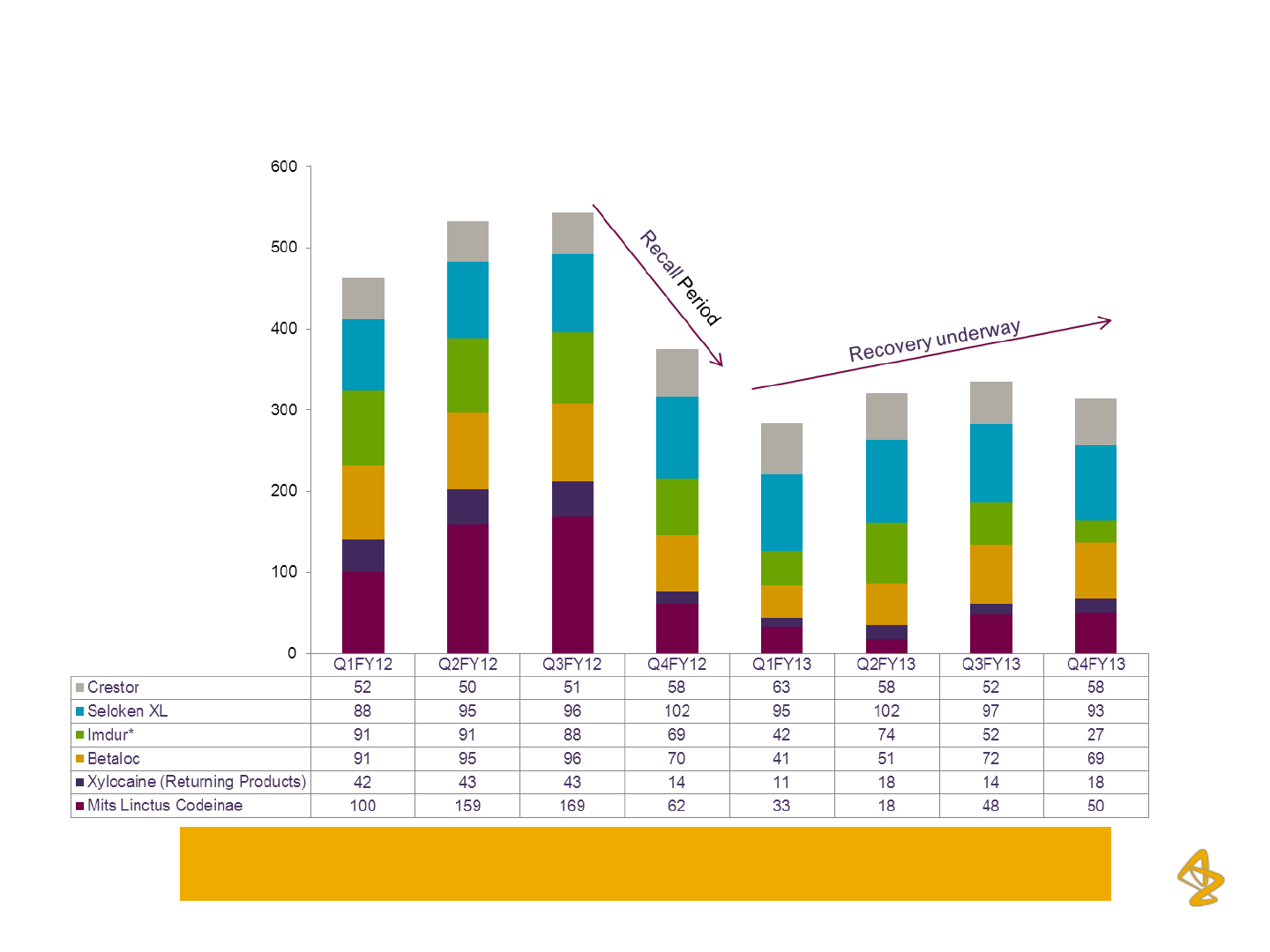
AZPIL: Steady Recovery Under-way
Sales for Impacted Brands (In Rs. million)
Remediation of Supply Issues enabling recovery across Key Impacted Brands
*Imdur sales were impacted between Q2FY13 and Q4FY13 on account of certain supply issues, which are currently being remediated
Products representing 8 – 10% of the revenues are being discontinued in the
current financial year (FY14)
15%
24%
24%
30%
29%
32%
34%
24%
18%
7%
-14%
7%
14%
13%
18%
18%
20%
20%
14%
11%
4%
-22%
-30%
-20%
-10%
0%
10%
20%
30%
40%
2002 2003 2004 2005 2006 2007 2008 2009 FY11 FY12 FY13
EBITDA Margin PAT Margin
1,401
1,808
1,965
2,329
2,774
3,136
3,681
4,024
6,003
5,379
4,009
0
1,000
2,000
3,000
4,000
5,000
6,000
7,000
2002 2003 2004 2005 2006 2007 2008 2009 FY11 FY12 FY13
217
432
481
700
807
1,012
1,261
946
1,098
366
-580
-1,000
-500
0
500
1,000
1,500
2002 2003 2004 2005 2006 2007 2008 2009 FY11 FY12 FY13
104
245
258
431
487
615
738
576
641
198
-895
-1,000
-800
-600
-400
-200
0
200
400
600
800
1,000
2002 2003 2004 2005 2006 2007 2008 2009 FY11 FY12 FY13
27
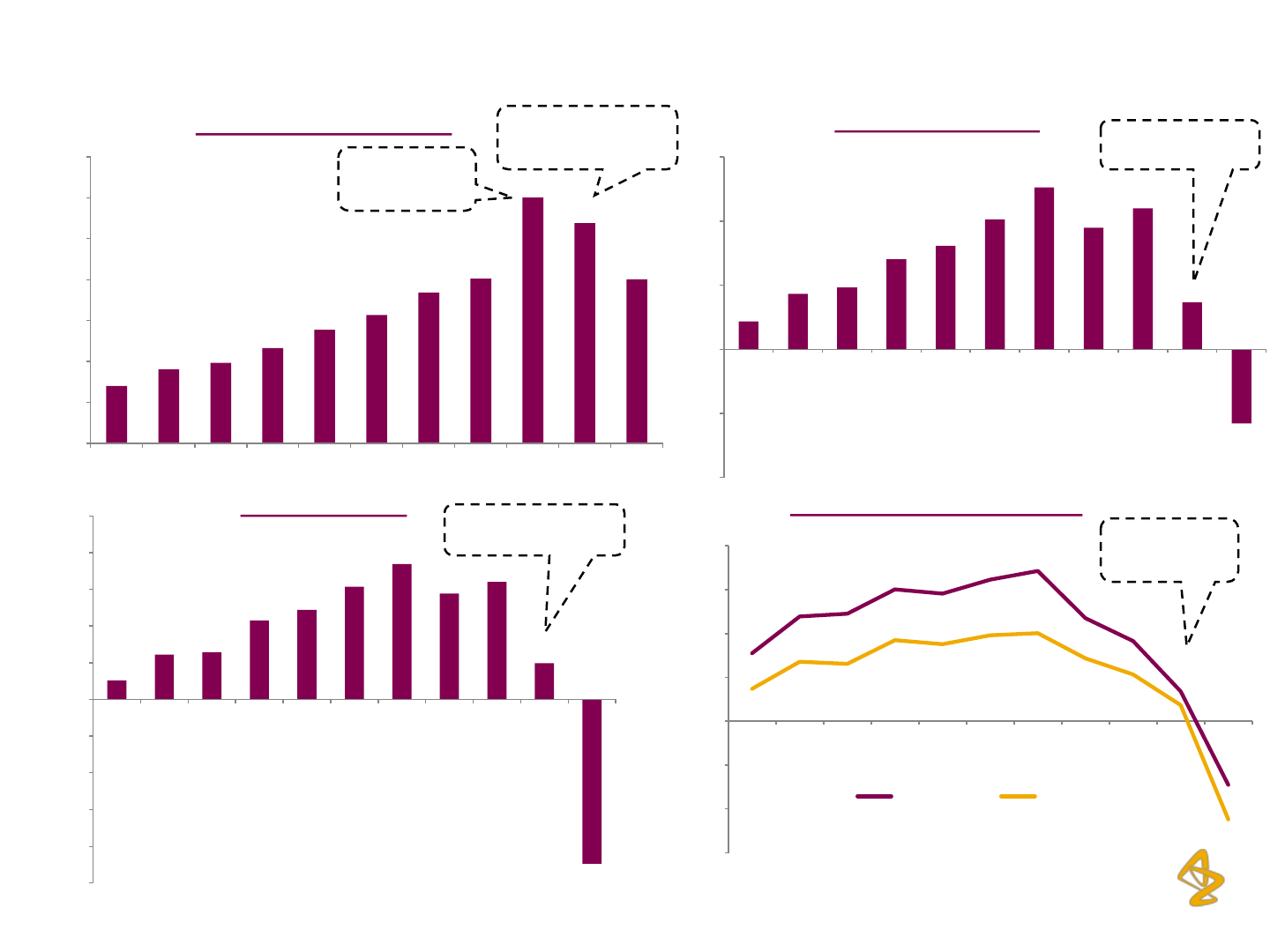
AZPIL: Financial Overview
27
Total Income (Rs. million)
EBITDA (Rs. million)
EBITDA and PAT Margins (%)
PAT (Rs. million)
Voluntary recall
initiated
2002-11 CAGR 22.1%
Voluntary
recall initiated
2002-11 CAGR 19.1%
15 month
period FY11
2002-11 CAGR 16.7%
Voluntary recall
initiated
Voluntary
recall
initiated
28
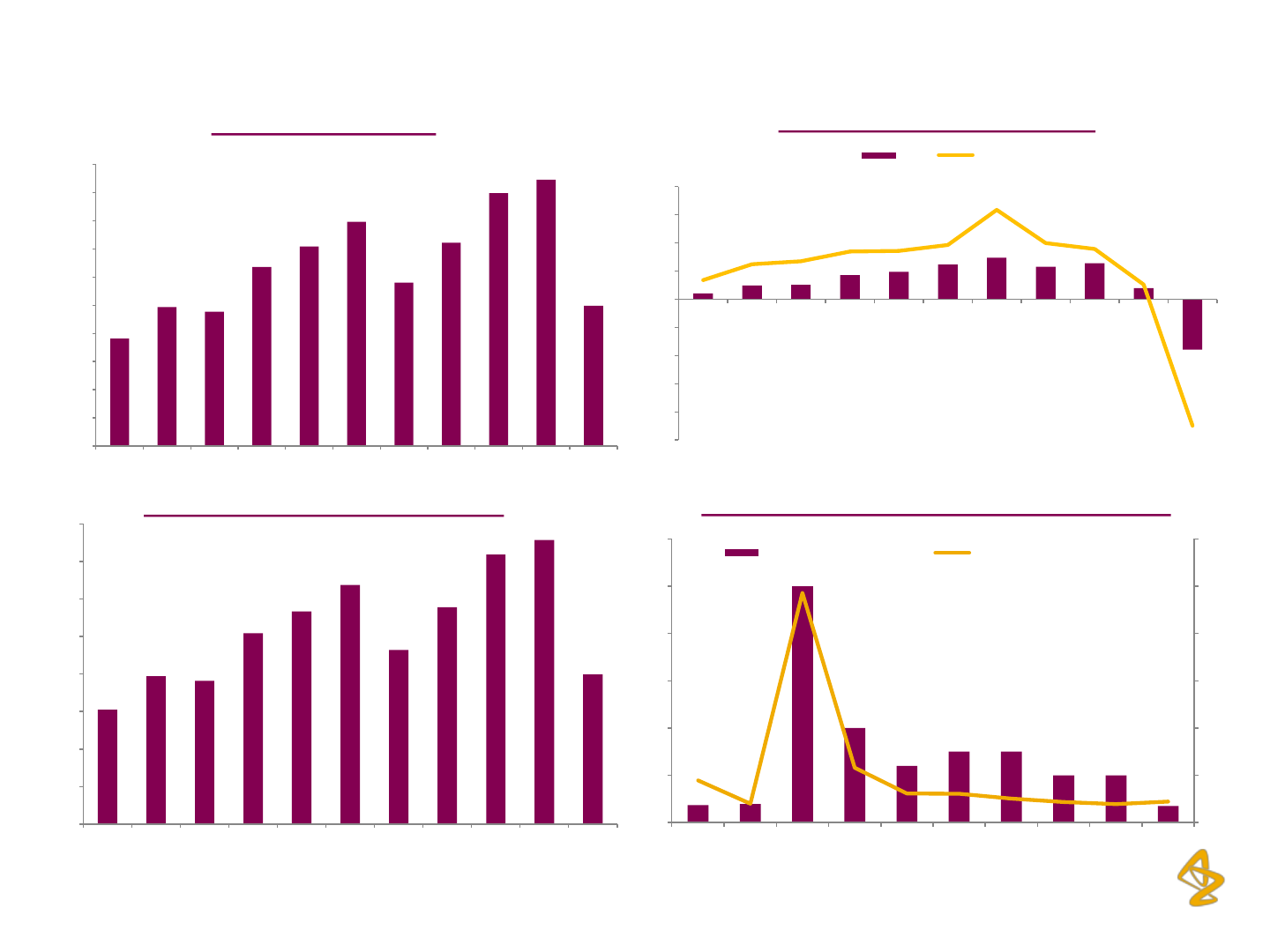
Net worth (Rs. million)
EPS (Rs.) / Return on Net worth
Dividend Per Share/ Dividend Payout Ratio (%)
Book Value Per Share (BVPS in Rs.)
3.7
3.9
50
20
12
15 15
10 10
3.5
89
40
485
116
62
61
51
43
39
44
0
100
200
300
400
500
600
0
10
20
30
40
50
60
2002 2003 2004 2005 2006 2007 2008 2009 FY11 FY12
Dividend Per Share (INR) Dividend Payout Ratio- RHS (%)
AZPIL: Financial Overview
762
987
955
1,272
1,417
1,593
1,162
1,445
1,797
1,893
998
0
200
400
600
800
1,000
1,200
1,400
1,600
1,800
2,000
2002 2003 2004 2005 2006 2007 2008 2009 FY11 FY12 FY13
4
10
10
17
19
25
30
23
26
8
-36
14
25
27
34
34
39
64
40
36
10
-90
-100
-80
-60
-40
-20
0
20
40
60
80
2002 2003 2004 2005 2006 2007 2008 2009 FY11 FY12 FY13
EPS RoNW
30
39
38
51
57
64
46
58
72
76
40
0
10
20
30
40
50
60
70
80
2002 2003 2004 2005 2006 2007 2008 2009 FY11 FY12 FY13
29 29
3 AZPIL: Key Highlights
Overview
30
AZPIL: Key Investment Highlights
30
Leveraging Global Strength
Outstanding Parent profile providing
significant technical & financial backing;
Consistent product launches from Parent’s
portfolio
Significant Growth Levers
Favorably positioned to benefit from India’s
fast growing Pharma market
Re-focused for Delivery
Resolution of Supply Issues in India and
taking steps towards its strengthening
position in the Indian Market
Strong Pipeline Potential
Significant launches planned in India from
global portfolio and partnerships.
High Corporate Governance
Standards
High corporate governance;
Passion for quality and patient safety ,
demonstrated by initiating voluntary recall
based on findings of AstraZeneca Worldwide
Audit Group’s (WWAG) quality audit
Long Term Commitment to India
Among the first MNCs to establish India
presence; Focus on India in-line with Global
strategy to ramp-up business in emerging
markets
31 31
AZPIL: Leveraging Global Strength
Product launches from Parent’s portfolio
2002 2003 2004 2005 2006 2007 2008 2009 FY11 FY12 FY13
Cardiovascular
Crestor Brilinta
Respiratory
Symbicort
Turbuhaler
Antibiotic/
Infection
Meronem
Relaunch
of Diprivan
Oncology
Zoladex
Arimidex
Zoladex
(10.8 mg)
Casodex
Faslodex
Relaunch
of
Nolvadex
Iressa
Anaesthesia
Diprivan
Maternal
Healthcare
Gastro Intestinal
Neksium
Diabetes
Alliance
Onglyza
Kombiglyze
XR
32 32
Robust Global Pipeline
AZD2014
moxetumo
mab* AZD4547 MEDI-551* lesinurad
brodalumab
*
volitinib*
MEDI0639
* Olaparib
#
tremelimum
ab
fostamati
nib* metreleptin*
AZD1208
MEDI3617
*
selumetinib
* MEDI-573*
naloxegol
*
AZD9150 MEDI-565* AZD5069
benralizum
ab* CAZ AVI*
AZD8330*
MEDI6469
* AZD2115*
mavrilimum
ab*
AZD5363*
MEDI4736
* AZD5423* MEDI8968*
AZD8848* MEDI4212 AZD1722*
sifalimuma
b*
AZD7594*
MEDI2070
* AZD6765 MEDI-546*
AZD7624
MEDI9929
* AZD5213
Tralokinum
ab
AZD1446*
MEDI5872
* AZD3241 MEDI7183*
AZD3293* MEDI5117 AZD5847
ATM AVI MEDI-557
MEDI-559
MEDI-550
Oncology
Respiratory &
Inflammation
CVMD
Neuroscience
Infection
Legend
Phase I
26 NMEs
Phase II
21 NMEs
Phase III/
Registration
6 NMEs
AZPIL: Strong Pipeline Potential
Significant technical support and robust Global Pipeline & launches
from collaborations offers significant future growth visibility
World Class R&D and Technology Support
Investment >$4 billion each year
9,800 Employees
Spread across 10 principal R&D centers in 6 countries
Collaborated with different companies including Bristol-Myers
Squibb & Amgen and acquisitions like Ardea Biosciences,
MedImmune & Amylin Pharmaceuticals (in alliance with BMS),
which has further augmented current R&D pipeline, with
approximately 40% being sourced externally
Launched five of its global blockbuster products in the Indian
Market
Access to global technical knowledge and Product portfolio
R&D Support
Technology
Support
#
Decision to accelerate
Olaparib to Phase III,
and committed to EU
filing in 2013
33
Domestic Formulations Market (in Rs. Bn) Split by therapeutic segment
Source: Industry Research
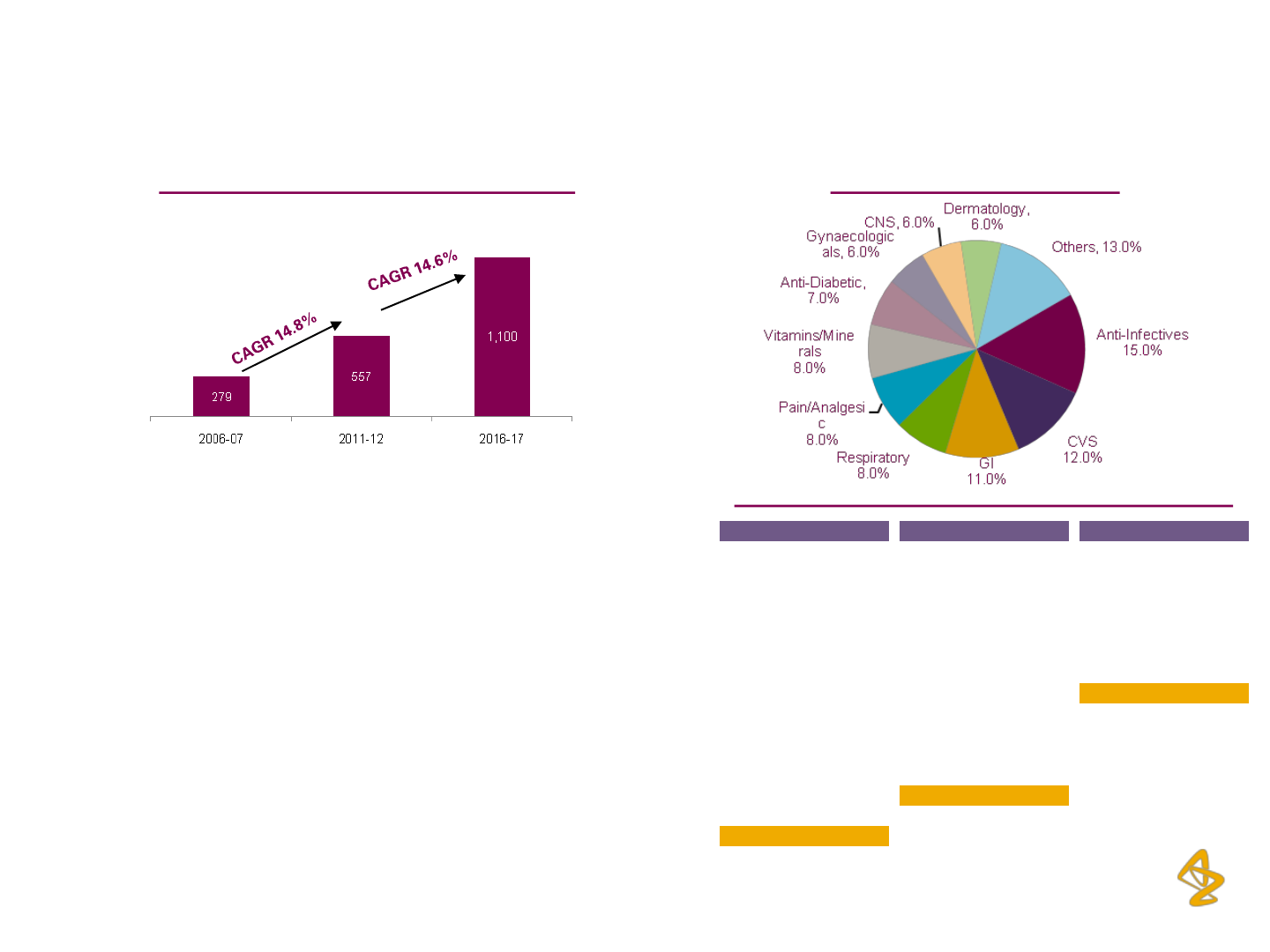
Expected CAGR of 14 - 17% over the next 5 years with
the market size expected to cross Rs. 1 trillion
High growth expected in Specialty therapies (Diabetes,
Oncology, CVS, CNS, among others); Mass therapies
such as anti-Infective and Gastro segments also
expected to continue growing steadily
Top 10 therapies have remained constant over the last 5
years, consistently contributing more than 85% of the
market in value terms
2006 Rankings
1 United States
2 Japan
3 France
4 Germany
5 China
6 Italy
7 Spain
8 UK
9 Canada
10 Brazil
11 Australia
12 Mexico
13 South Korea
14 Russia
15 India
India’s improving ranking in Global Pharma Space
2011 Rankings
1 United States
2 Japan
3 China
4 Germany
5 France
6 Brazil
7 Italy
8 Spain
9 Canada
10 UK
11 Russia
12 Australia
13 India
14 South Korea
15 Mexico
2016 Rankings
1 United States
2 China
3 Japan
4 Brazil
5 Germany
6 France
7 Italy
8 India
9 Russia
10 Canada
11 UK
12 Spain
13 Australia
14 Argentina
15 South Korea
Ranking in all years based on spending in constant US$ at Q4
2011 exchange rates
Source: IMS Market Prognosis, May 2012
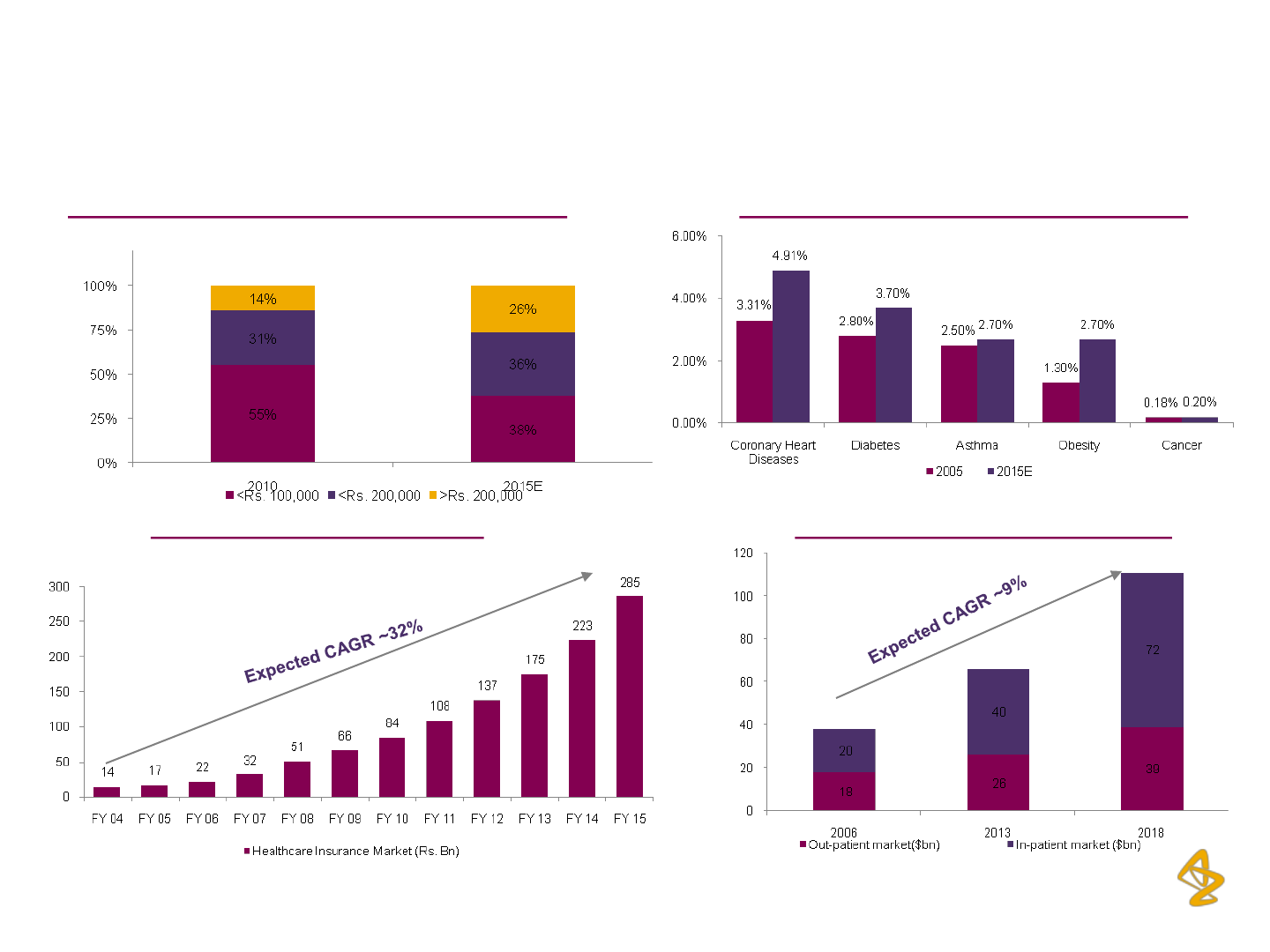
AZPIL: Significant Growth Levers
Favorably positioned to benefit from India’s fast growing Pharma Market
34
Household incomes* to drive healthcare spending Rising prevalence of several chronic diseases
Expanding healthcare delivery market Growing health insurance market
Source: HealthCare Market Research
AZPIL: Significant Growth Levers
Favorably positioned to benefit from India’s fast growing Pharma Market
Percent of Population
* Annual income

35
Favorable product mix of AZPIL
Catering to majority segments of the Indian Pharma Market
AZPIL: Significant Growth Levers
Favorably positioned to benefit from India’s fast growing Pharma Market
Though traditionally, acute therapies have dominated the Indian pharma market, holding a significantly higher share
than chronic segments, but with changing lifestyles and demographics, the disease profile of the Indian population is
shifting towards chronic ailments, leading to faster growth in chronic segment (CAGR of 17-19% over FY09-12 as
compared to the CAGR of 11-14% recorded by the acute segment during the same period)
AZPIL’s portfolio is favorably positioned to capture this re-alignment in the Indian Pharma market from acute
therapies to chronic therapies, over the long term
AZPIL is also considering select marketing tie-ups for some of the products, in order to improve their market
penetration and revenues
0% 20% 40% 60% 80% 100%
Sanofi India
Astrazeneca India
Abbott India
IPM
Novartis India
Pfizer India
Wyeth India
Glaxo India
Acute Chronic
Therapeutic Segments
(Rs. billion) 2006-07 2011-12E 2016-17P
Anti Diabetic 12.4 36.5 80 – 87
Anti Infectives 49.7 91.3 147 – 161
CVS 28.2 66.7 128 – 140
Dermatology 15.5 31.0 62 – 68
Gastro Intestinal 30.5 59.2 109 – 119
Gynaecologicals 15.2 31.5 58 – 61
Neuro / CNS 15.0 31.4 63 – 69
Pain / Analgesics 26.3 46.4 82 – 89
Respiratory 25.8 47.3 83 – 91
Vitamins / Minerals 23.8 44.4 85 – 93
Others 36.4 70.9 125 – 137
Total 279.0 556.6 1050 – 1100
Source: Industry Research; E – Estimated; P – Projected; Therapeutic segments
where AZPIL has presence have been highlighted
36
AZPIL: High Corporate Governance Standards
Emphasis on consistent global standards of sales and marketing practices
Maintaining a strong focus on patient safety
Exploring ways of increasing access to healthcare for more people, tailored locally to different
patient needs
High Operating
Values & Ethics
AZPIL’s Board has historically been composed of non-executive and independent directors, with
the promoter representative directors forming the rest
AZPIL’s Chairman is a Non-Executive and Independent director, Mr. D. E. Udwadia, who is an M.A
L.L.B. (Hons.) by qualification and has over 48 years of active corporate law practice and wide-
ranging professional experience; He is a solicitor and advocate of the Bombay High Court, and
solicitor of the Supreme Court of England, and brings with him significant legal expertise
Robust Board
Composition
Stringent and superior global quality and manufacturing standards
Undertook a voluntary recall of sterile products manufactured at its Bengaluru plant, following
AstraZeneca Worldwide Audit Group’s (WWAG) quality audit in Q4FY12
As a precautionary measure, also voluntarily suspended production temporarily to review
manufacturing and quality practices at the plant, and undertake remedial measures
Resulted in near-term adverse financial impact, but was done in the overall interests of the Indian
consumers and doctor fraternity, in line with its global best practices
Upholds High Corporate Governance standards of the AstraZeneca Group
Voluntary Recall
upholds Global
Quality and
Corporate
Governance
Standards
AZPIL: Re-focused for Delivery
Re-Focused to deliver products and performance
37
To launch products suitable for the Indian market from its global portfolio and alliance partners,
which shall augment the existing product portfolio
Launching Global
Products in India
State of the art manufacturing facility being established in Bengaluru, Karnataka with capacity to
manufacture 1.2 billion tablets, expected to commence operations from Q1FY2014
Commencement of
New Tablet Facility
Reintroduction of
products
Manufacturing of injectables and liquids are being outsourced by AZPIL to selected contract
manufacturers in India
Outsourcing
Prior to the recall, AZPIL was one of the fastest growing MNC pharma companies in India
On account of the recall, AZPIL faced certain supply issues in FY2013, which are in the process of
being remediated and addressed, with key products like Xylocaine (Injection), Prostodin, Seloken
XL and Sensorcaine already being re-introduced in the market
AZPIL: Re-focused for Delivery
Re-alignment of Manufacturing Strategy
Existing Formulations Manufacturing Facility
Tablet Manufacturing Facility
The new tablet manufacturing facility is expected to commence commercial production in Q1FY2014
As on March 31, 2013, Rs. 737 million has been incurred towards the manufacturing facility, and an additional Rs.
80 million is proposed to be incurred before commencement of commercial production
Further, additional investment of Rs. 188 million has been approved towards the manufacturing facility, which
shall be spent over FY14-FY15
API Facility
Agreement with AZAB Sweden for supply of TBS over the next three years
38
The existing manufacturing facility is proposed to be closed by end FY14, once the new tablet manufacturing
facility commences production, the technology transfer of products is completed and the production stabilizes
This would allow us to consolidate our operations leaving approximately 30 acres of land unutilized
39
AZPIL: Long-Term Commitment to India
In-line with the Global Emerging Market Strategy
39
Establishing New state-of-the-art manufacturing facility with an investment of Rs 1,005 million
Significant Human capital investment as well, in terms of bringing experienced personnel within
the group to manage the commencement and stabilization of the new manufacturing facility
Voluntary non-repayable financial grant by the promoter AstraZeneca Pharmaceuticals AB
Sweden of approximately USD 22.5 mio (Rs. 1,192 mio) to USD 26.5 mio (Rs. 1,404 mio) over the
three years period FY14 – FY16 under a subvention agreement, of which the first tranche of USD
14 mio (Rs. 740 mio) shall be provided to the company in the current financial year FY14
Investment
Commitment
India remains a key strategic growth market for the AZ group, among the emerging markets
Finds mention as one of the fastest growing markets for the Group
Presents significant potential in terms of AZ’s diabetes alliance product portfolio, on
account of the significant diabetic population
Strategic Growth
Market for AZ
Global
Present in India for more than 34 years
Existing formulations manufacturing facility commenced commercial production in 1982 and has
been supplying products for the Indian and global markets for more than 30 years
Among the earliest MNC pharma companies to enter the Indian market
Long-standing
presence in India
The voluntary recall initiated by AZPIL is indicative of AstraZeneca group’s commitment to
providing high quality medicines, and ensuring patient safety, while demonstrating its long term
commitment to the Indian market
Voluntary recall
Initiative
AZPIL: Going Forward
40
Company’s revenues to grow ahead of market over this period
Re-establish its market position in the impacted products portfolio
Return to profitability in the current financial year and deliver PBT margin in
high teens from the next financial year
The above should be read together with the underlying assumptions given in slide 41
National Pharmaceuticals Pricing Policy (NPPP), if implemented could have
an adverse impact of 10 – 15% to the sales of AZPIL
AZPIL: Assumptions Underlying Way Forward
Strong performance of key AZPIL brands and products
Remediation Strategy remains on track without any further supply issues, both in-house and from
outsourcing suppliers
Ability to overcome any resistance in re-establishing the impacted products back in market
No delay in new product launches on account of regulatory approvals / any other reasons
Restructuring exercise completed smoothly within estimated timelines and budgeted costs
Commencement of new tablet manufacturing facility along expected timelines and within the
estimated costs, and stabilization of the facility in line with current plans
Internal Factors
and Business
Environment
No Adverse impact of any other macro-economic & Industry / Company specific developments
and any unforeseen circumstance
General Risk
The Indian Pharma market continues to grow in double digits over the next 2-3 years
There are no adverse changes in the proposed NPPP (incremental), Patent product pricing,
Pharma Marketing Policy, Patent Act or significant shift in government view on Product Patents
There are no adverse changes in Government policies on import of medicines in bulk or tablet
form and those on clinical trials or associated compensation rules
There are no adverse changes in custom or import duties
The Government continues to provide an un-interrupted supply of Codeinae
There is no adverse impact of generic entry in the patented products space
Industry Outlook
and Regulatory
Environment
41

Thank You

